
Ŀǰ������60%��þ�ǴӺ�ˮ����ȡ�ġ���֪��ˮ��ȡþ����Ҫ�������£�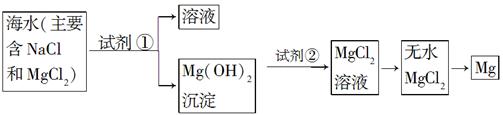
��1�����ڼ����Լ������������������¼��ֲ�ͬ������������������⡣
| ���� | �Ƿ���ȷ | �������� |
| ����1��ֱ������ˮ�м�������� | ����ȷ | ��һ�� |
| ����2�����¼���������ˮ���ټ�������� | ������ | ������ |
| ����Ϊ����������������ǣ����������ģ� | ||
 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д� ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ڿ������ױ����������γɸ��ο��ȶ����ڡ����������[(NH4)2SO4?FeSO4?6H2O]��һ����Ҫ��ѧ�Լ���ʵ���ҿ��ô���Ƭ����ȡ��������泥��������£�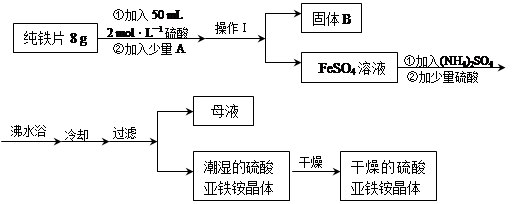
��ش��������⣺
��1��������������� ��������Ҫ��������Ʒ�У�����̨����Ȧ���⣩ ��
��2����A����ΪCuSO4��������Ŀ���� �������B�ijɷ�Ϊ ��
��3��������������Ե���pHΪ 1��2���������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������һ����Ҫ�Ļ���ԭ�ϡ�Ŀǰ�Ƽҵ��Ҫ�С�������͡������Ƽ�����ֹ��ա��밴Ҫ��ش����⣺
(1)���������������CaCl2�������д���ù����в���CaCl2�Ļ�ѧ����ʽ��___________________________��
(2)д���������Ƽ���йط�Ӧ�Ļ�ѧ����ʽ��_______________________
(3)CO2���Ƽҵ����Ҫԭ�ϣ��������Ƽ���롰�������CO2����Դ�кβ�ͬ��_______________________��
(4)��ɫ��ѧ����Ҫԭ��֮һ����߷�Ӧ��ԭ�������ʡ����ݡ������Ƽ���ܷ�Ӧ�г�����ԭ�������ʵı���ʽ��ԭ��������(%)��_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ�����Ʊ�����ͭ����[Cu(HCOO)2��4H2O]���������£�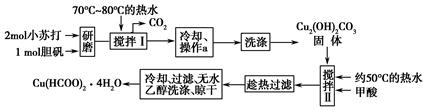
�ش��������⣺
(1)С�մ�ˮ��Һ��________��(��ᡱ��������С�)������θ��(0.2%��0.4%������)���࣬��Ӧ�����ӷ���ʽΪ________________________��
(2)���ɼ�ʽ̼��ͭ[Cu2(OH)2CO3]�����ӷ���ʽΪ________________________��
(3)����a��������________���ò���ʹ�õIJ���������________��
(4)֤����ʽ̼��ͭ�Ѿ�ϴ�ӳ�ֵ�ʵ�������________________________________��
(5)����ˮ�Ҵ�ϴ�Ӿ����Ŀ����___________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����������Ļ�����Ӧ�ù㷺����FeCl3������������ӡˢ��·ͭ�帯ʴ��������ֹѪ���ȡ�
��1��д��FeCl3��Һ��ʴӡˢ��·ͭ������ӷ���ʽ��
________________________________________________________________��
��2��������1���еķ�Ӧ��Ƴ�ԭ��أ��뻭��ԭ��ص�װ��ͼ�����������������д���缫��Ӧʽ��
������Ӧ�� _________________________________________��
������Ӧ��__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ʯ��һ�ָ�þ�����ο�����ܳƣ�������ɫ������������Ƥ������������ʯ���Կ�����MgO��FeO��Fe2O3��Al2O3��SiO2��ɡ���ҵ��������ʯ��ȡ��ʽ̼��þ��Ʒ���������£�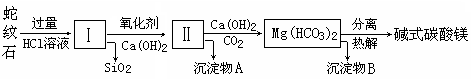
��1������ʯ�������ܽ����Һ�����Mg2����Al3+�⣬�����еĽ���������________��
��2������м����������������� ������Ca(OH)2ʱ��
��Ҫ������ҺpH��7��8֮��(�й��������������pH���±�)��
| �������� | Fe(OH)3 | Al(OH)3 | Mg(OH)2 |
| ��ʼ����pH | 1.5 | 3.3 | 9.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Ϊ�˽��͵��������Ի������ɵ�Ӱ�죬��һ����������·������õ���70��Cu��25��Al��4��Fe������Au��Pt�Ƚ����Ļ�������Ƴ������Ʊ�����ͭ�������������·�ߣ�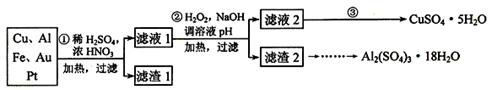
��1���ڢٲ�Cu����ᷴӦ�����ӷ���ʽΪ______________________________���õ�����1����Ҫ�ɷ�Ϊ_________________��
��2���ڢڲ��м���H2O2��������__________________��ʹ��H2O2���ŵ���_________������ҺpH��Ŀ����____________________________________��
��3�������ڢ۲�����Һ2�õ�CuSO4��5H2O�ķ�����_________________________
____________________________________________________________ ��
��4��������2��ȡAl2(SO4)3��18H2O ��������������ַ�����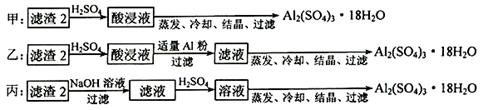
�������ַ����У�_______���������У�ԭ����_______________________________��
��ԭ�������ʽǶȿ��ǣ�_______������������
��5���õζ����ⶨCuSO4��5H2O������ȡa g�������100 mL��Һ��ÿ��ȡ20.00 mL�������������Ӻ���c mol��L-1 EDTA��H2Y2��������Һ�ζ����յ㣬ƽ������EDTA��Һb mL���ζ���Ӧ���£�Cu2+ + H2Y2���� CuY2�� + 2H+��д������CuSO4��5H2O���������ı���ʽ�أ� __________________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ����ij����(����Cu2O��Al2O3��Fe2O3��SiO2)��ȡͭ�IJ����������£�
��֪��Cu2O��2H��=Cu��Cu2����H2O
(1)ʵ������������Ϊ________���ڿ��������չ�������Dʱ���õ����ֹ������ʵ������������������ƾ��ơ��������⣬����________(����������)��
(2)��ҺA����Ԫ�صĴ�����ʽΪ________(�����ӷ���)�����ɸ����ӵ����ӷ���ʽΪ__________________��������ҺA�д��ڸ����ӵ��Լ�Ϊ________(���Լ�����)��
(3)��������E���������F������ijһ��Ӧ�����ں��Ӹֹ죬�÷�Ӧ�Ļ�ѧ����ʽΪ_______________________________________________��
(4)�����£���pH��NaAlO2��NaOH������Һ�У���ˮ�������c(OH��)ǰ��Ϊ���ߵ�108������������Һ��pH��________��
(5)�����õ�ⷨ���д�ͭ����ʱ������������ȷ����________(�����)��
a������ȫ��ת��Ϊ��ѧ��
b����ͭ�ӵ�Դ����������������Ӧ
c����ͭ��������������Һ��Cu2��Ũ�ȼ�С
d����ͭ����ʱͨ���ĵ�������������ͭ������ȷ����ϵ
�ڴ�Ũ���ᡢŨ���ᡢ����ˮ��ѡ�ú��ʵ��Լ����ⶨ��ͭ��Ʒ�н���ͭ�������������漰����Ҫ���裺��ȡһ����������Ʒ��________________�����ˡ�ϴ�ӡ����������ʣ�����ͭ��������(��ȱ�ٵIJ������裬���������������̵�ϸ��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��14�֣� ij��ѧС��ģ�⡰�����Ƽ������NaCl��NH3��CO2��ˮ��Ϊԭ���Լ���ͼ��ʾװ
ij��ѧС��ģ�⡰�����Ƽ������NaCl��NH3��CO2��ˮ��Ϊԭ���Լ���ͼ��ʾװ
����ȡNaHCO3����Ӧ�Ļ�ѧ����ʽΪNH3+CO2+H2O+NaCl = NaHCO3��+NH4Cl��Ȼ���ٽ�NaHCO3�Ƴ�
Na2CO3��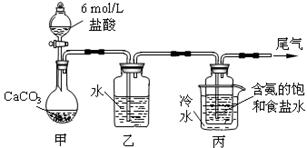
��1��װ���ҵ������� ��Ϊ��ֹ��Ⱦ������β���к��е� ��Ҫ�������մ�����
��2����װ�ñ��в�����NaHCO3��ȡNa2CO3ʱ����Ҫ���е�ʵ������� �� �� ��
NaHCO3ת��ΪNa2CO3�Ļ�ѧ����ʽΪ ��
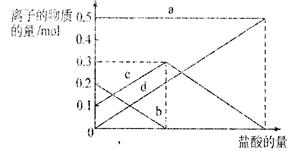
��3�����ڣ�2�������յ�ʱ��϶̣�NaHCO3���ֽⲻ��ȫ����С���һ�ݼ�����t1 min��NaHCO3 ��Ʒ����ɽ���������̽����
ȡ������t1 min��NaHCO3��Ʒ29.6 g��ȫ����ˮ�Ƴ���Һ��Ȼ�������Һ�л����صμ�ϡ���ᣬ�����Ͻ��衣��������ļ��룬��Һ���й����ӵ����ʵ����ı仯����ͼ��ʾ��
������c��Ӧ����Һ�е�������
�������ӷ��ţ�������Ʒ��NaHCO3��Na2CO3�����ʵ���֮���� ��
��4����ȡ21. 0 g NaHCO3���壬������t2 min��ʣ����������Ϊl4.8 g������Ѵ�ʣ�����ȫ�����뵽200 mL 2 mol��L-1�����������ַ�Ӧ����Һ��H+ �����ʵ���Ũ��Ϊ____________������Һ����仯���Բ��ƣ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com