
Ϊ�˽��͵��������Ի������ɵ�Ӱ�죬��һ����������·������õ���70��Cu��25��Al��4��Fe������Au��Pt�Ƚ����Ļ�������Ƴ������Ʊ�����ͭ�������������·�ߣ�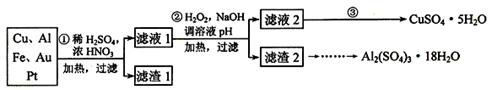
��1���ڢٲ�Cu����ᷴӦ�����ӷ���ʽΪ______________________________���õ�����1����Ҫ�ɷ�Ϊ_________________��
��2���ڢڲ��м���H2O2��������__________________��ʹ��H2O2���ŵ���_________������ҺpH��Ŀ����____________________________________��
��3�������ڢ۲�����Һ2�õ�CuSO4��5H2O�ķ�����_________________________
____________________________________________________________ ��
��4��������2��ȡAl2(SO4)3��18H2O ��������������ַ�����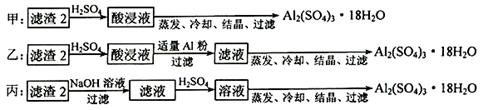
�������ַ����У�_______���������У�ԭ����_______________________________��
��ԭ�������ʽǶȿ��ǣ�_______������������
��5���õζ����ⶨCuSO4��5H2O������ȡa g�������100 mL��Һ��ÿ��ȡ20.00 mL�������������Ӻ���c mol��L-1 EDTA��H2Y2��������Һ�ζ����յ㣬ƽ������EDTA��Һb mL���ζ���Ӧ���£�Cu2+ + H2Y2���� CuY2�� + 2H+��д������CuSO4��5H2O���������ı���ʽ�أ� __________________ ��
��1��Cu + 4H+ + 2NO3- =Cu2+ + 2NO2��+ 2H2O��
3Cu + 8H+ + 2 NO3-=3Cu2+ + 2NO��+ 4H2O��2�֣���Au��Pt��1�֣�
��2����Fe2+����ΪFe3+ ��1�֣� ���������ʣ��Ի�������Ⱦ��1�֣���
ʹFe3+��Al3+������ȥ��2�֣�
��3��������Һ2��������������ȴ�� �ᾧ������ �������Ƶ�����ͭ���壨2�֣�
��4���� �����ò�Ʒ�к��н϶�Fe2(SO4)3���ʣ� �� ����1�֣���3�֣�
��5�� �� 100% ��2�֣�
�� 100% ��2�֣�
���������������1��Cu����ᷴӦ��ʵ������H+��NO3-��Ӧ���淴Ӧ��������Ũ����С���������ӷ���ʽΪCu + 4H+ + 2NO3- =Cu2+ + 2NO2��+ 2H2O��3Cu + 8H+ + 2 NO3-=3Cu2+ + 2NO��+ 4H2O��Au��Pt������ᷴӦ��������������Ҫ�ɷ���Au��Pt��
��2���ӹ��������Ŀ���ǰ��������������������ӣ������ȥ�����Ҽ���������ⲻ�������µ�����������Ⱦ��������Һ��pHĿ����ʹFe3+��Al3+������ȥ
��3������Һ2�õ�CuSO4��5H2O�ķ����ǰ���Һ������Ũ��Һ����ȴ�ᾧ�����˵�����ͭ����
��4�����������У���Ϊ����2����Ҫ�ɷ���Fe��OH��3��Al��OH��3�����������������ȫ���ܽ�ʹ�ƵõIJ�Ʒ�к��н϶�Fe2(SO4)3���ʣ� ��ԭ�������ʽǶȷ������ҷ����������������ܳ�ȥ��������ͬʱ������������������ԭ�������ʽϸ�
��5���ɵζ���Ӧ����ʽ��100ml��Һ��n��Cu2+��=b��10-3��a��5mol,����CuSO4��5H2O��������= b��10-3��a��5��250/a��100%
���㣺������ѧ��������Ԫ�����漰���Ʊ������ӡ�ʵ�顢����֪ʶ



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��CΪ��ѧ�������ʣ�����һ��Ϊ������ͨ������£�AΪ���壬BΪҺ�壬C
Ϊ���塣D��E��F��G��H��X��Ϊ���������X��һ������ǿ�ᡢEΪ��ɫ���壬H�ڳ�����ΪҺ�塣����֮���ת����ϵ��ͼ��ʾ(����ijЩ��Ӧ�����Ͳ��ַ�Ӧ������ȥ)��
(1)д����ѧʽ��A________��D________��E________��X________��
(2)�ڷ�Ӧ�١����У�������������ԭ��Ӧ����________(����)��
(3)��Ӧ�����ӷ���ʽΪ��_______________________________________��
(4)��Ӧ�ߵĻ�ѧ����ʽΪ_________________________________________��
�÷�Ӧ��ÿ����0.3 mol��A����ת�Ƶ���______mol��
(5)д��D����Һ��С�մ���Һ��Ӧ�����ӷ���ʽ��_________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������Ԫ��A��B��C��Dλ��ͬ������ԭ����������������֪A��C��D����Ԫ��ԭ�ӵ�����㹲��10�����ӣ���������Ԫ�ص��������������Ӧ��ˮ����֮���������ܷ�Ӧ���������κ�ˮ����������и�С�⣺
(1)A��B��C���������ӵİ뾶�ɴ�С��˳���ǣ�____>_____>____(��д���ӷ���)��
(2)ָ��Ԫ��D��Ԫ�����ڱ��е�λ�ã���________���ڵ�________�塣
(3)Ԫ��B������������________������(����ӡ����ۡ�)�������ʽΪ________________��
(4)A��CԪ�ص��������������Ӧ��ˮ����֮�䷴Ӧ�����ӷ���ʽΪ________________��
(5)����C�����ӵ��γ���������ˮ����ԭ��Ϊ______________(�����ӷ���ʽ��ʾ���ʵ�������˵��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Ŀǰ������60%��þ�ǴӺ�ˮ����ȡ�ġ���֪��ˮ��ȡþ����Ҫ�������£�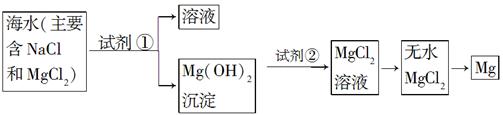
��1�����ڼ����Լ������������������¼��ֲ�ͬ������������������⡣
| ���� | �Ƿ���ȷ | �������� |
| ����1��ֱ������ˮ�м�������� | ����ȷ | ��һ�� |
| ����2�����¼���������ˮ���ټ�������� | ������ | ������ |
| ����Ϊ����������������ǣ����������ģ� | ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ�����������᳧����(��Ҫ�ɷ�Ϊ���������P����FeS��SiO2��)�Ʊ�����(��ʽ�������ľۺ���)���̷�(FeSO4��7H2O)���������£�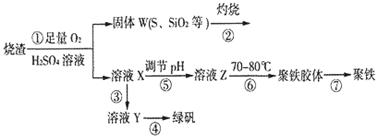
��1�������̢��еIJ���������ͨ��������Һ�У���Һ����ɫ����__________��
A��Ʒ����Һ B����ɫʯ����Һ C������KMnO4��Һ D����ˮ
��2�����̢��У�FeS��O2��H2SO4��Ӧ�Ļ�ѧ����ʽΪ��___________________________________��
��3�����̢��У�������������___________________________��
��4�����̢��У������ᾧ��Ҫʹ�þƾ��ơ����Ǽܡ������ǣ�����Ҫ��������_______________��
��5�����̢ݵ���pH��ѡ�������Լ��е�___________ (��ѡ�����)��
A��ϡ���� B��CaCO3 C��NaOH��Һ
��6�����̢��У�����ҺZ���ȵ�70һ80�棬Ŀ����_____________________��
��7��ʵ����Ϊ�������õ��ľ�����Ʒ����Ԫ�ص�������������������ʵ�顣���÷�����ƽ��ȡ2.70g��Ʒ���ڽ���Ʒ�����������������������Ȼ�����Һ���۹��ˡ�ϴ�ӡ�����������ù�������Ϊ3.495g�����þ�����Ҫ�ɷ�Ϊ[(Fe(OH)(SO4)]n����þ�����Ʒ����Ԫ�ص���������Ϊ___________��(���������в�����Ԫ�غ���Ԫ��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͭ��CuFeS2������ȡͭ���仯�������Ҫԭ��֮һ�������Ʊ�������Ļ����
��1��ұ��ͭ�ķ�ӦΪ�� 8CuFeS2��21O2 8Cu��4FeO��2Fe2O3��16SO2
8Cu��4FeO��2Fe2O3��16SO2
��CuFeS2��Fe�Ļ��ϼ�Ϊ��2����Ӧ�б���ԭ��Ԫ����________����Ԫ�ط��ţ���
��2������ұ�����̲�������SO2�����д��������к�������________������ţ���
a���߿��ŷ�
b�������Ʊ�����
c���ô�����Һ������Na2SO3
d����Ũ��������
��3�����û�ͭ��ұ��ͭ������¯������Fe2O3��FeO��SiO2��Al2O3�����Ʊ�Fe2O3������Ϊ��
����ϡ�����ȡ¯�������ˡ�
����Һ���������ټ������NaOH��Һ�����ˣ�������ϴ�ӡ�������յ�Fe2O3��
����������Ϣ�ش��������⣺
a����ȥAl3�������ӷ���ʽ��______________________��
b��ѡ���ṩ���Լ������ʵ����֤¯���к���FeO��
�ṩ���Լ���ϡ���ᡡϡ���ᡡKSCN��Һ��KMnO4��Һ��NaOH��Һ����ˮ
��ѡ�Լ�Ϊ______________��֤��¯���к���FeO��ʵ������Ϊ______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ȷ�Ӧ������ұ�����۵�Ľ������ɼ���Ϊ������ijЩ�����������ڸ��������·����ķ�Ӧ��ijѧϰС������ȷ�Ӧ����Al��Fe2O3��ӦΪ����ʵ������о���
�������ݵõ�Al��Al2O3��Fe��Fe2O3���۵㡢�е��������±���ʾ��
| ���� | Al | Al2O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2054 | 1535 | 1565[ |
| �е�/�� | 2467 | 2980 | 2750 | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������ҵ���������ɡ���������ȡ������ұ�������ļӹ��Ȼ��ڹ��ɡ���ش���������:
��1����ҵ�ϲ��õ��������-����ʯ(Na3AlF6)������ķ���ұ���õ�������:
2Al2O3 4Al+3O2���������ʯ������:_______________________________��
4Al+3O2���������ʯ������:_______________________________��
��2��������������������������������Fe��Si������,���õ�ⷽ����һ���ᴿ,�õ����������ĵ缫��ӦʽΪ������ ��,���п����������ϵ���________________��
A.���ġ� ��B.ʯī�� ��C.Ǧ�塡�� D.����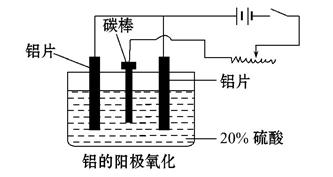
��3������������ʹ���������������ܵ�����Ĥ����ϡ����Ϊ���Һ,�����������ĵ缫��ӦʽΪ__________________________________��
��4��������������������,��Ҫ���ϵص�����ѹ,������____________ ��
��5������˵����ȷ��������������
A. ����������Ӧ��ԭ���ԭ�����н������ϱ��洦���ļ���
B. ����������������ǿ������ľ�Ե����
C. ����������������߽���������Ͻ����ʴ��,����ĥ���½�
D. ������������Ĥ���ж����,���к�ǿ����������,������Ⱦ�϶��ʸ�����ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ի�ͭ����Ҫ�ɷ�ΪCuFeS2������������SiO2�ȣ�Ϊԭ�Ͻ�����ͭ��ͬʱ�õ�����Ʒ�̷���FeSO4��7H2O��������Ҫ�������£�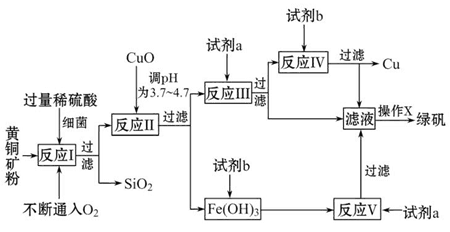
��֪���� 4CuFeS2+2H2SO4+17O2=4CuSO4+2Fe2��SO4��3+2H2O
�ڲ���������������������ʽ��ʼ��������ȫ����ʱ��Һ��pH���
| ������ | Cu��OH��2 | Fe��OH��3 | Fe��OH��2 |
| ��ʼ����pH | 4��7 | 2��7 | 7��6 |
| ��ȫ����pH | 6��7 | 3��7 | 9��6 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com