
��������ҵ���������ɡ���������ȡ������ұ�������ļӹ��Ȼ��ڹ��ɡ���ش���������:
��1����ҵ�ϲ��õ��������-����ʯ(Na3AlF6)������ķ���ұ���õ�������:
2Al2O3 4Al+3O2���������ʯ������:_______________________________��
4Al+3O2���������ʯ������:_______________________________��
��2��������������������������������Fe��Si������,���õ�ⷽ����һ���ᴿ,�õ����������ĵ缫��ӦʽΪ������ ��,���п����������ϵ���________________��
A.���ġ� ��B.ʯī�� ��C.Ǧ�塡�� D.����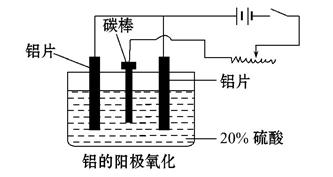
��3������������ʹ���������������ܵ�����Ĥ����ϡ����Ϊ���Һ,�����������ĵ缫��ӦʽΪ__________________________________��
��4��������������������,��Ҫ���ϵص�����ѹ,������____________ ��
��5������˵����ȷ��������������
A. ����������Ӧ��ԭ���ԭ�����н������ϱ��洦���ļ���
B. ����������������ǿ������ľ�Ե����
C. ����������������߽���������Ͻ����ʴ��,����ĥ���½�
D. ������������Ĥ���ж����,���к�ǿ����������,������Ⱦ�϶��ʸ�����ɫ
��1������Al2O3�۵� ��2�֣�
��2��Al- 3e-= Al3+��2�֣� D��2�֣�
��3��2 Al- 6e-+ 3H2O = Al2O3 + 6H+��2�֣�
��4�����������治�������������������Ϊ�˱����ȶ��ĵ�������Ҫ���������ѹ����3�֣�
��5��B��D��4�֣�
���������������1���������۵�ϸߣ����ʱ�ܺĴ������ʯ���Խ���Al2O3�ۻ����¶ȣ������ܺģ���2����Ͽα���ѧ����ͭ�ƾ�ͭ����ͭ����������ͭ��������֪����������������������Ӧ���缫����ʽΪ;Al- 3e-= Al3+,������������ѡ��D�𰸣���3��������������������Ӧ�γ����������缫��ӦΪ��2Al- 6e-+ 3H2O = Al2O3 + 6H+����4�����������治�������������������Ϊ�˱����ȶ��ĵ�������Ҫ���������ѹ����5��B��D
���㣺����绯ѧԭ�����缫����ʽ����д��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������һ����Ҫ�Ļ���ԭ�ϡ�Ŀǰ�Ƽҵ��Ҫ�С�������͡������Ƽ�����ֹ��ա��밴Ҫ��ش����⣺
(1)���������������CaCl2�������д���ù����в���CaCl2�Ļ�ѧ����ʽ��___________________________��
(2)д���������Ƽ���йط�Ӧ�Ļ�ѧ����ʽ��_______________________
(3)CO2���Ƽҵ����Ҫԭ�ϣ��������Ƽ���롰�������CO2����Դ�кβ�ͬ��_______________________��
(4)��ɫ��ѧ����Ҫԭ��֮һ����߷�Ӧ��ԭ�������ʡ����ݡ������Ƽ���ܷ�Ӧ�г�����ԭ�������ʵı���ʽ��ԭ��������(%)��_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Ϊ�˽��͵��������Ի������ɵ�Ӱ�죬��һ����������·������õ���70��Cu��25��Al��4��Fe������Au��Pt�Ƚ����Ļ�������Ƴ������Ʊ�����ͭ�������������·�ߣ�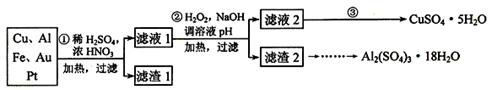
��1���ڢٲ�Cu����ᷴӦ�����ӷ���ʽΪ______________________________���õ�����1����Ҫ�ɷ�Ϊ_________________��
��2���ڢڲ��м���H2O2��������__________________��ʹ��H2O2���ŵ���_________������ҺpH��Ŀ����____________________________________��
��3�������ڢ۲�����Һ2�õ�CuSO4��5H2O�ķ�����_________________________
____________________________________________________________ ��
��4��������2��ȡAl2(SO4)3��18H2O ��������������ַ�����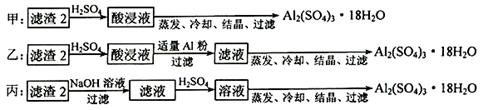
�������ַ����У�_______���������У�ԭ����_______________________________��
��ԭ�������ʽǶȿ��ǣ�_______������������
��5���õζ����ⶨCuSO4��5H2O������ȡa g�������100 mL��Һ��ÿ��ȡ20.00 mL�������������Ӻ���c mol��L-1 EDTA��H2Y2��������Һ�ζ����յ㣬ƽ������EDTA��Һb mL���ζ���Ӧ���£�Cu2+ + H2Y2���� CuY2�� + 2H+��д������CuSO4��5H2O���������ı���ʽ�أ� __________________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ����ij����(����Cu2O��Al2O3��Fe2O3��SiO2)��ȡͭ�IJ����������£�
��֪��Cu2O��2H��=Cu��Cu2����H2O
(1)ʵ������������Ϊ________���ڿ��������չ�������Dʱ���õ����ֹ������ʵ������������������ƾ��ơ��������⣬����________(����������)��
(2)��ҺA����Ԫ�صĴ�����ʽΪ________(�����ӷ���)�����ɸ����ӵ����ӷ���ʽΪ__________________��������ҺA�д��ڸ����ӵ��Լ�Ϊ________(���Լ�����)��
(3)��������E���������F������ijһ��Ӧ�����ں��Ӹֹ죬�÷�Ӧ�Ļ�ѧ����ʽΪ_______________________________________________��
(4)�����£���pH��NaAlO2��NaOH������Һ�У���ˮ�������c(OH��)ǰ��Ϊ���ߵ�108������������Һ��pH��________��
(5)�����õ�ⷨ���д�ͭ����ʱ������������ȷ����________(�����)��
a������ȫ��ת��Ϊ��ѧ��
b����ͭ�ӵ�Դ����������������Ӧ
c����ͭ��������������Һ��Cu2��Ũ�ȼ�С
d����ͭ����ʱͨ���ĵ�������������ͭ������ȷ����ϵ
�ڴ�Ũ���ᡢŨ���ᡢ����ˮ��ѡ�ú��ʵ��Լ����ⶨ��ͭ��Ʒ�н���ͭ�������������漰����Ҫ���裺��ȡһ����������Ʒ��________________�����ˡ�ϴ�ӡ����������ʣ�����ͭ��������(��ȱ�ٵIJ������裬���������������̵�ϸ��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ҹ���ѧ�Һ�°�ĸ����Ĵ�����������,�������̿ɼ�Ҫ��ʾ��ͼ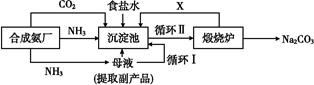
(1)������������ķ�������������,����Ʒ��һ����;Ϊ����������
(2)�������з����Ļ�ѧ��Ӧ����ʽ�� ����
(3)д������������X���ʵķ���ʽ�� ��
(4)ʹԭ���Ȼ��Ƶ������ʴ�70%��ߵ�90%����,��Ҫ���������������(�����������еı��)��ѭ�����ӳ�������ȡ�������IJ������������� ��
(5)Ϊ�����Ʒ̼�������Ƿ����Ȼ���,��ȡ������������ˮ��,�ٵμ�����������
(6)��ĸҺ��ͨ����,����ϸСʳ�ο���,��ȴ��������Ʒ,ͨ����������������������
a.����NH4+��Ũ��,ʹNH4Cl���������
b.ʹNaHCO3���������
c.ʹNaHCO3ת��ΪNa2CO3,���������NH4Cl����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������г��������᳧���ջ�����ʯ������(��Ҫ�ɷ�Ϊ���������P����FeS��SiO2��)���Ʊ�����(��ʽ�������ľۺ���)���̷�(FeSO4��7H2O)�������� �£�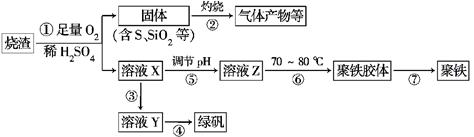
(1)�����̢��в���������ͨ��������Һ�У���Һ����ɫ����________(��ѡ�����)��
a��Ʒ����Һ b����ɫʯ����Һ c������KMnO4��Һ d����ˮ
(2)���̢��У�FeS��O2��H2SO4��Ӧ�����ӷ���ʽΪ______________________
(3)���̢��У�������������____________����Ӧ�Ļ�ѧ����ʽΪ_____________________
����ҺY���̷�ʱ����ȡ����Y��Һ���Թ��У����Թ��ڼ���������________________��Һ���۲���Һ�Ƿ��Ϊ________ɫ������֤�����Ƿ���Fe3����
(4)��ʵ�������ɹ��̢��е�____________(���������)����Ҫʹ�þƾ��ơ����żܡ�����ǯ�ȣ�����Ҫ�IJ���������___________________________��
(5)���̢��У�����ҺZ���ȵ�70��80�棬Ŀ����____________________________________________________��
(6)ʵ����Ϊ�������õ��ľ�����Ʒ����Ԫ�ص�������������������ʵ�飺���÷�����ƽ��ȡ2.700 g��Ʒ���ڽ���Ʒ�����������������������Ȼ�����Һ���۹��ˡ�ϴ�ӡ�����������ù�������Ϊ3.495 g�����þ�������Ҫ�ɷ�Ϊ[Fe(OH)SO4]n���������Ʒ����Ԫ�ص���������Ϊ________��(���������в�����Ԫ�غ���Ԫ��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ת����ϵ�У�X��Y����������;�㷺�����ֽ������ʣ�A��B�������A�ʺ���ɫ��C��D��E����ѧ���������ֻ��������ת����ϵ�ش����⣺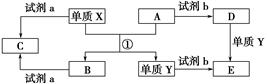
��1����д����Ӧ�ٵĻ�ѧ����ʽ___________________________________________��
��2������D��Һ��Y���ӵķ�����_____________________________________��
��3�����Լ�a��NaOH��Һ��д������X��NaOH��Һ��Ӧ�����ӷ���ʽ______________________________��
��4�����Լ�b��H2SO4����ҵ����E��H2SO4��NaNO2Ϊԭ����ȡ��Ч��ˮ��Y��OH��SO4����֪��ԭ����ΪNO����÷�Ӧ�Ļ�ѧ����ʽ��_____________________________________________________________________��
��5����ҵ�ϵ�����ڵ�B��ȡXʱ�������������������ڱ�״���µ����Ϊ33.6 m3�����������������Ϊ________kg��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��14�֣� ij��ѧС��ģ�⡰�����Ƽ������NaCl��NH3��CO2��ˮ��Ϊԭ���Լ���ͼ��ʾװ
ij��ѧС��ģ�⡰�����Ƽ������NaCl��NH3��CO2��ˮ��Ϊԭ���Լ���ͼ��ʾװ
����ȡNaHCO3����Ӧ�Ļ�ѧ����ʽΪNH3+CO2+H2O+NaCl = NaHCO3��+NH4Cl��Ȼ���ٽ�NaHCO3�Ƴ�
Na2CO3��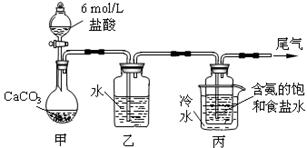
��1��װ���ҵ������� ��Ϊ��ֹ��Ⱦ������β���к��е� ��Ҫ�������մ�����
��2����װ�ñ��в�����NaHCO3��ȡNa2CO3ʱ����Ҫ���е�ʵ������� �� �� ��
NaHCO3ת��ΪNa2CO3�Ļ�ѧ����ʽΪ ��
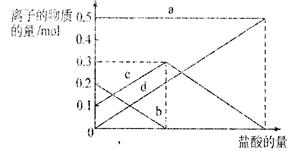
��3�����ڣ�2�������յ�ʱ��϶̣�NaHCO3���ֽⲻ��ȫ����С���һ�ݼ�����t1 min��NaHCO3 ��Ʒ����ɽ���������̽����
ȡ������t1 min��NaHCO3��Ʒ29.6 g��ȫ����ˮ�Ƴ���Һ��Ȼ�������Һ�л����صμ�ϡ���ᣬ�����Ͻ��衣��������ļ��룬��Һ���й����ӵ����ʵ����ı仯����ͼ��ʾ��
������c��Ӧ����Һ�е�������
�������ӷ��ţ�������Ʒ��NaHCO3��Na2CO3�����ʵ���֮���� ��
��4����ȡ21. 0 g NaHCO3���壬������t2 min��ʣ����������Ϊl4.8 g������Ѵ�ʣ�����ȫ�����뵽200 mL 2 mol��L-1�����������ַ�Ӧ����Һ��H+ �����ʵ���Ũ��Ϊ____________������Һ����仯���Բ��ƣ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��EΪ��ѧ��ѧ�����ĵ��ʻ�����ת����ϵ����ͼ��ʾ��
��1����A�ǵ���ɫ�����������D����ɫ���壻C�к��е����������Ӿ�Ϊ10�������ӡ�
��д����ӦA��B�Ļ�ѧ����ʽ ��
�ڽ�һ����������Dͨ��2 L C����Һ�У���������Һ�б���μ���ϡ����������������������������������ʵ����Ĺ�ϵ��ͼ������������ܽ��HCl�Ļӷ�������ش�O����Һ���������ʵĻ�ѧʽΪ ��a����Һ�и�����Ũ���ɴ�С�Ĺ�ϵ�� ��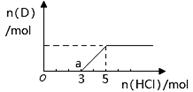
��2��������1���е�����Dͨ������������Һ�еò�����F��F��KSP=8.1��10-9���ֽ��ó�������0.1mol/L ��BaCl2��Һ�У���KSP ���������С�� �䣩����ɲ�����F������������Һ�е�Ũ��Ϊ mol/L��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com