
A��B��C��D��EΪ��ѧ��ѧ�����ĵ��ʻ�����ת����ϵ����ͼ��ʾ��
��1����A�ǵ���ɫ�����������D����ɫ���壻C�к��е����������Ӿ�Ϊ10�������ӡ�
��д����ӦA��B�Ļ�ѧ����ʽ ��
�ڽ�һ����������Dͨ��2 L C����Һ�У���������Һ�б���μ���ϡ����������������������������������ʵ����Ĺ�ϵ��ͼ������������ܽ��HCl�Ļӷ�������ش�O����Һ���������ʵĻ�ѧʽΪ ��a����Һ�и�����Ũ���ɴ�С�Ĺ�ϵ�� ��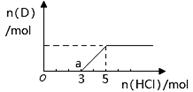
��2��������1���е�����Dͨ������������Һ�еò�����F��F��KSP=8.1��10-9���ֽ��ó�������0.1mol/L ��BaCl2��Һ�У���KSP ���������С�� �䣩����ɲ�����F������������Һ�е�Ũ��Ϊ mol/L��
��1��2CO2 + 2Na2O2�� 2Na2CO3 + O2 (3��)NaOH��Na2CO3 (2��)
c(Na+)��c(Cl-)��c(HCO3-)��c(OH-)��c(H+)��c(CO32-) (3��)
��2������ (2��) �� 8.1��10-8 (2��)
��������������������Ϲ�ϵͼȷ��AΪ�������ƣ�BΪ̼���ƣ�CΪ�������ƣ�����A��B��ѧ����ʽΪ2CO2 + 2Na2O2�� 2Na2CO3 + O2����̼���������ᷴӦ���ص�Na2CO3+HCl=NaHCO3+NaCl��NaHCO3+HCl=NaCl+H2O+CO2������������ͬ�������ᣬ����ȷ��ͼ��O�������ΪNaOH��Na2CO3 ��a ��Ϊ3molNaCl��2molNaHCO3,����Ũ�ȴ�СΪ c(Na+)��c(Cl-)��c(HCO3-)��c(OH-)��c(H+)��c(CO32-) ��Kspֻ���¶�Ӱ�죬���Բ��䣬��c��SO42-��=Ksp��BaSO4��/c(Ba2+)=8.1��10-8.
���㣺�ƵĻ����


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������ҵ���������ɡ���������ȡ������ұ�������ļӹ��Ȼ��ڹ��ɡ���ش���������:
��1����ҵ�ϲ��õ��������-����ʯ(Na3AlF6)������ķ���ұ���õ�������:
2Al2O3 4Al+3O2���������ʯ������:_______________________________��
4Al+3O2���������ʯ������:_______________________________��
��2��������������������������������Fe��Si������,���õ�ⷽ����һ���ᴿ,�õ����������ĵ缫��ӦʽΪ������ ��,���п����������ϵ���________________��
A.���ġ� ��B.ʯī�� ��C.Ǧ�塡�� D.����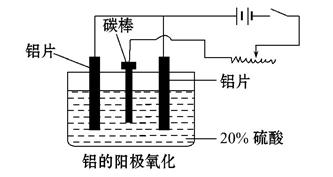
��3������������ʹ���������������ܵ�����Ĥ����ϡ����Ϊ���Һ,�����������ĵ缫��ӦʽΪ__________________________________��
��4��������������������,��Ҫ���ϵص�����ѹ,������____________ ��
��5������˵����ȷ��������������
A. ����������Ӧ��ԭ���ԭ�����н������ϱ��洦���ļ���
B. ����������������ǿ������ľ�Ե����
C. ����������������߽���������Ͻ����ʴ��,����ĥ���½�
D. ������������Ĥ���ж����,���к�ǿ����������,������Ⱦ�϶��ʸ�����ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ի�ͭ����Ҫ�ɷ�ΪCuFeS2������������SiO2�ȣ�Ϊԭ�Ͻ�����ͭ��ͬʱ�õ�����Ʒ�̷���FeSO4��7H2O��������Ҫ�������£�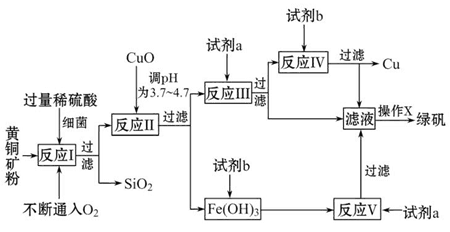
��֪���� 4CuFeS2+2H2SO4+17O2=4CuSO4+2Fe2��SO4��3+2H2O
�ڲ���������������������ʽ��ʼ��������ȫ����ʱ��Һ��pH���
| ������ | Cu��OH��2 | Fe��OH��3 | Fe��OH��2 |
| ��ʼ����pH | 4��7 | 2��7 | 7��6 |
| ��ȫ����pH | 6��7 | 3��7 | 9��6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��13�֣�ij�����A������KAl(SO4)2��Al2O3��Fe2O3����һ�������¿�ʵ����ͼ��ʾ������֮��ı仯��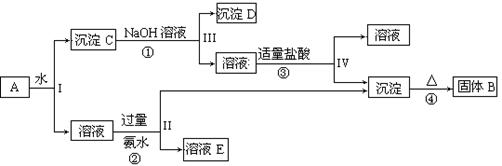
�ݴ˻ش��������⣺
��1��I��II��III��IV�IJ��ж�����Һ�ͳ����ķ����ȡ�ķ����� ��
��2������������ͼ��Ӧ��ϵ��д������B�� D�������ʵĻ�ѧʽ
����B ������D ��
��3��д���١��ڷ�Ӧ�����ӷ���ʽ��
�� ���� ��
��4����Fe2O3Ϊԭ�ϣ����Ʊ�FeCl2��Һ����д���йصĻ�ѧ��Ӧ����ʽ���Լ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ú��������������������ͭ��ȡ�Ȼ�ͭ����(CuCl2 xH2O)�������²�����
xH2O)�������²�����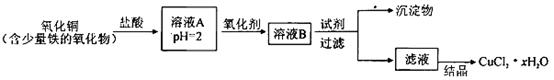
��֪����pHΪ4��5ʱ��Fe3��������ȫˮ�������������ʱCu2��ȴ������ˮ�⡣
(1)��ҺA�еĽ���������Fe3����Fe2����Cu2����������ҺA��Fe2��������Լ�Ϊ_____�����ţ���ͬ����
��KMnO4 ��(NH4)2S ��NaOH �� KSCN
(2)��������ѡ��_________����Cl2 ��KMnO4 ��HNO3 ��H2O2
(3)Ҫ�õ��ϴ��IJ�Ʒ���Լ���ѡ��_______________����NaOH ��FeO ��CuO ��Cu2(OH)2CO3
(4)����Һ�����ᾧ�õ��Ȼ�ͭ����ķ�����_____________����ʵ���Ⱥ�˳��ѡ���ţ���
�ٹ��� ������Ũ�� ���������� ����ȴ
(5)���ⶨ��ҺA�е�Fe2����Ũ�ȣ�ʵ��ǰ������Ҫ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250mL������ʱ��Ҫ����������ƽ�����������ձ���ҩ�ס���ͷ�ι��⣬����_________�����еζ���ʽ�У����������___________���гֲ�����ȥ��������ĸ��ţ���
(6)Ϊ�˲ⶨ�Ƶõ��Ȼ�ͭ���壨CuCl2 xH2O���е�xֵ��ijѧ�����������ʵ�鷽����
xH2O���е�xֵ��ijѧ�����������ʵ�鷽����
����һ����ȡmg�����������������ټ���Ϊֹ����ȴ������������ˮCuCl2������Ϊn1g
����������ȡmg��������ˮ��������������������Һ�����ˡ�����ϴ�Ӻ���С��������������ټ���Ϊֹ����ȴ���������ù��������Ϊn2g��
��������������ʵ�鷽����������ȷ�ķ�����________��������___________���ݴ˼����x��_________���ú�m��n1��n2�Ĵ���ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��E��F�������ʵ���ɫ��Ӧ��Ϊ��ɫ�� A��B��C��D�����ᷴӦ������E������B�����ɡ��ֿ�ȼ�����壻��C��D�����ɡ�����ɫ��ζ������H����������ʹ�����ʯ��ˮ����ǡ�D��A�ɷ�Ӧ����C��F��HҲ�ɷ�Ӧ����C����������ɫ��ζ���塣
��ش��������⣺
��1��д��B��C�Ļ�ѧʽ��B��___________________; C��___________________;
��2��д��F��H2O��Ӧ�Ļ�ѧ����ʽ��__________________________________;
��3��д�����з�Ӧ�����ӷ���ʽ
��D��Һ+���_____________________________________________________;
��D��Һ+A��Һ��___________________________________________________;
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����Fe��Cu��ɵĺϽ�����Cu��Fe�������ʵ���Ϊa mol��Cu�����ʵ�������Ϊx���гɷ�ĩ��ȫ��Ͷ�뺬b mol HNO ��ϡ��Һ�У���ʹ���ַ�Ӧ��������Ļ�ԭ����ֻ��NO���Իش��������⣺
��ϡ��Һ�У���ʹ���ַ�Ӧ��������Ļ�ԭ����ֻ��NO���Իش��������⣺
��1����ʣ��IJ���ֻ��Cu������Һ�к��еĽ�������Ϊ__________��(д�����п������)
��2������Һ�н�������ֻ��Fe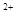 ��Cu
��Cu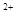 ʱ����b��ȡֵ��Χ��(��a��x��ʾ)___________��
ʱ����b��ȡֵ��Χ��(��a��x��ʾ)___________��
��3����x��0.5����Һ��Fe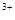 ��Fe
��Fe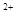 �����ʵ������ʱ���ڱ�״���¹�����672 mL���塣��a��____________��b��_____________��
�����ʵ������ʱ���ڱ�״���¹�����672 mL���塣��a��____________��b��_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����һ��Ӧ�ù㷺�Ľ�������ҵ����Al2O3�ͱ���ʯ��Na3AlF6��������ڵ���Ƶá�
�����������Ҫ�ɷ���Al2O3��SiO2�ȡ���������������Al2O3���������£�
����өʯ��CaF2���ʹ���Ϊԭ���Ʊ�����ʯ���������£�
�ش��������⣺
��1��д����Ӧ1�Ļ�ѧ����ʽ ��
��2����Һ���м���CaO���ɵij����� ����Ӧ2�����ӷ���ʽΪ ��
��3��E����Ϊ�������ϣ�������C�� ��д����D�Ʊ�����ʯ�Ļ�ѧ����ʽ ��
��4����������Ļ�ѧ����ʽ�� ����ʯīΪ�缫�����������Ļ������ijɷ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪A��B��C��DΪ�������ʣ�����B��C��D���³�ѹ��Ϊ���壬�ס��ҡ�������Ϊ������ҳ�����ΪҺ�壬������ɫ��ӦΪ��ɫ����ͼΪ��������֮������Ӧ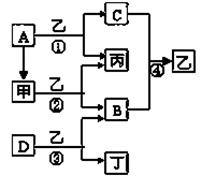
��1��д���������ʵĻ�ѧʽ��
A ��B ��D ���� ��
��2��������ʽΪ ����Ӧ��������11��2L����״���£�B���ɣ�����ת�Ƶĵ�
�ӵ����ʵ���Ϊ ��
��3��д����Ӧ�۵Ļ�ѧ����ʽ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com