
����ת����ϵ�У�X��Y����������;�㷺�����ֽ������ʣ�A��B�������A�ʺ���ɫ��C��D��E����ѧ���������ֻ��������ת����ϵ�ش����⣺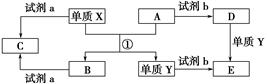
��1����д����Ӧ�ٵĻ�ѧ����ʽ___________________________________________��
��2������D��Һ��Y���ӵķ�����_____________________________________��
��3�����Լ�a��NaOH��Һ��д������X��NaOH��Һ��Ӧ�����ӷ���ʽ______________________________��
��4�����Լ�b��H2SO4����ҵ����E��H2SO4��NaNO2Ϊԭ����ȡ��Ч��ˮ��Y��OH��SO4����֪��ԭ����ΪNO����÷�Ӧ�Ļ�ѧ����ʽ��_____________________________________________________________________��
��5����ҵ�ϵ�����ڵ�B��ȡXʱ�������������������ڱ�״���µ����Ϊ33.6 m3�����������������Ϊ________kg��
 ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������Ԫ��A��B��C��Dλ��ͬ������ԭ����������������֪A��C��D����Ԫ��ԭ�ӵ�����㹲��10�����ӣ���������Ԫ�ص��������������Ӧ��ˮ����֮���������ܷ�Ӧ���������κ�ˮ����������и�С�⣺
(1)A��B��C���������ӵİ뾶�ɴ�С��˳���ǣ�____>_____>____(��д���ӷ���)��
(2)ָ��Ԫ��D��Ԫ�����ڱ��е�λ�ã���________���ڵ�________�塣
(3)Ԫ��B������������________������(����ӡ����ۡ�)�������ʽΪ________________��
(4)A��CԪ�ص��������������Ӧ��ˮ����֮�䷴Ӧ�����ӷ���ʽΪ________________��
(5)����C�����ӵ��γ���������ˮ����ԭ��Ϊ______________(�����ӷ���ʽ��ʾ���ʵ�������˵��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ȷ�Ӧ������ұ�����۵�Ľ������ɼ���Ϊ������ijЩ�����������ڸ��������·����ķ�Ӧ��ijѧϰС������ȷ�Ӧ����Al��Fe2O3��ӦΪ����ʵ������о���
�������ݵõ�Al��Al2O3��Fe��Fe2O3���۵㡢�е��������±���ʾ��
| ���� | Al | Al2O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2054 | 1535 | 1565[ |
| �е�/�� | 2467 | 2980 | 2750 | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������ҵ���������ɡ���������ȡ������ұ�������ļӹ��Ȼ��ڹ��ɡ���ش���������:
��1����ҵ�ϲ��õ��������-����ʯ(Na3AlF6)������ķ���ұ���õ�������:
2Al2O3 4Al+3O2���������ʯ������:_______________________________��
4Al+3O2���������ʯ������:_______________________________��
��2��������������������������������Fe��Si������,���õ�ⷽ����һ���ᴿ,�õ����������ĵ缫��ӦʽΪ������ ��,���п����������ϵ���________________��
A.���ġ� ��B.ʯī�� ��C.Ǧ�塡�� D.����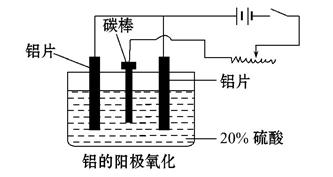
��3������������ʹ���������������ܵ�����Ĥ����ϡ����Ϊ���Һ,�����������ĵ缫��ӦʽΪ__________________________________��
��4��������������������,��Ҫ���ϵص�����ѹ,������____________ ��
��5������˵����ȷ��������������
A. ����������Ӧ��ԭ���ԭ�����н������ϱ��洦���ļ���
B. ����������������ǿ������ľ�Ե����
C. ����������������߽���������Ͻ����ʴ��,����ĥ���½�
D. ������������Ĥ���ж����,���к�ǿ����������,������Ⱦ�϶��ʸ�����ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ����������Ϊԭ���Ʊ��������ѵĹ�����������ͼ��ʾ�����������Ҫ�ɷ�Ϊ��������(FeTiO3)������һ������Ԫ���ڷ绯�����л�ת��Ϊ��3�ۡ�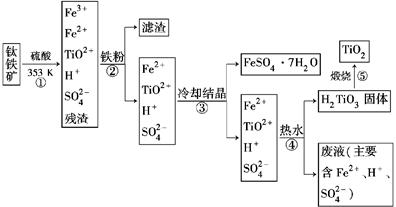
��֪��TiOSO4��ˮ��ˮ�⡣
(1)������У������۽�Fe3��ת��ΪFe2�������ӷ���ʽΪ_______________________
(2)������У�ʵ�ֻ����ķ������������ʵ�________(����ĸ���)��
a���ۡ��е���졡 b���ܽ��Բ��졡 c�������ԡ���ԭ�Բ���
(3)����ڡ��ۡ����У�����Ҫ���еIJ�����________(���������)��
(4)���ϻ�ѧ�����û�ѧƽ�����۽��Ͳ�����н�TiO2��ת��ΪH2TiO3��ԭ����
____________________________________________________________��
(5)�������������еķ�Һ�����̿�(��Ҫ�ɷ�ΪMnO2)��Ӧ������������(MnSO4��������ˮ)���÷�Ӧ�����ӷ���ʽΪ__________________________________
(6)�о����֣���ʯī������������������������CaF2��CaO������ʣ�������ͼ��ʾװ�ÿɻ�ý����ƣ������Ը�Ϊ��ԭ������ԭ���������Ʊ������ѡ�
д�������ĵ缫��Ӧʽ��_________________________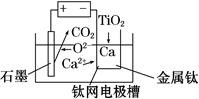
���Ʊ�������ǰ��CaO���������䣬��ԭ����______________________________________(���ϻ�ѧ�������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ˮ��һ�ַḻ����Դ����ҵ�ϴӺ�ˮ�п���ȡ���������ʣ��㷺Ӧ��������������Ƽ��ȷ��档��ͼ��ij�����Ժ�ˮ��Դ�����ۺ����õ�ʾ��ͼ��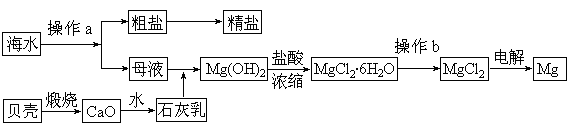
�ش��������⣺
��1������ͼ�в���a������Ϊ ��
��2����ҵ�ϴӺ�ˮ����ȡ��NaCl����������ȡ������Ҫ�������£���ʳ��ˮ����ͨ������A����ͨ������B����ַ�Ӧ����˵õ�����C����ҺD��������C���ռ����Ƶô��
����֪����A��B��CO2��NH3��������AӦ�� ���ѧʽ����
�����վ���C�ķ�Ӧ����ʽΪ ��
����ҺD����Ҫ����NH4Cl��NaHCO3�����ʣ���ҵ��������ҺD��ͨ��NH3��������ϸСʳ�ο�������ȴ������������NaHCO3�ĸ���ƷNH4Cl���壬��ͨ��NH3�������� ��
��3��þ��һ����;�ܹ�Ľ������ϣ�Ŀǰ������60%��þ�Ӻ�ˮ����ȡ��
�� ��Ҫ��֤������ˮMgCl2�в���NaCl����IJ��������ǣ� ��
�� ͨ������b�����ˮMgCl2���� ��Χ�н��У���ֱ���ڿ����м��ȣ��������Mg(OH)Cl��д���йط�Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij̽��С�齫һ����������·�������,�õ���70%Cu��25%Al��4%Fe������Au��Pt�Ƚ����Ļ����,����Ƴ������Ʊ�����ͭ�������������·��: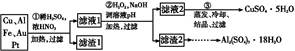
��ش���������:
��1���ڢٲ�Cu���ᷴӦ�����ӷ���ʽΪ�� ��
��2���ڢڲ���H2O2���������� ;
����ҺpH��Ŀ����ʹ�����������ɳ�����
��3���õڢ۲�����CuSO4��5H2O�Ʊ���ˮCuSO4�ķ���������������
��4��������2��ȡAl2��SO4��3��18H2O ,̽��С����������ַ���: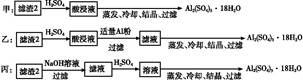
�������ַ�����,������������������,ԭ������ ������;
��ԭ�������ʽǶȿ���,��������������������
��5��̽��С���õζ����ⶨCuSO4��5H2O������ȡa g�������100 mL��Һ,ÿ��ȡ20.00 mL,�����������Ӻ�,��c mol /L EDTA��H2Y2-������Һ�ζ����յ�,ƽ������EDTA��Һb mL���ζ���Ӧ����:Cu2++H2Y2- CuY2-+2H+��д������CuSO4��5H2O���������ı���ʽw=�� ;
CuY2-+2H+��д������CuSO4��5H2O���������ı���ʽw=�� ;
���в����ᵼ��CuSO4��5H2O�����IJⶨ���ƫ�ߵ����������� ��
a.δ������ƿ
b.�ζ��յ�ʱ�ζ��ܼ����в�������
c.δ��������EDTA��Ӧ�ĸ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ի�ͭ����Ҫ�ɷ�ΪCuFeS2������������SiO2�ȣ�Ϊԭ�Ͻ�����ͭ��ͬʱ�õ�����Ʒ�̷���FeSO4��7H2O��������Ҫ�������£�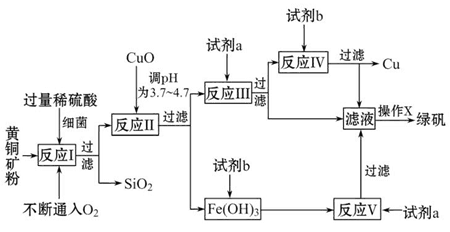
��֪���� 4CuFeS2+2H2SO4+17O2=4CuSO4+2Fe2��SO4��3+2H2O
�ڲ���������������������ʽ��ʼ��������ȫ����ʱ��Һ��pH���
| ������ | Cu��OH��2 | Fe��OH��3 | Fe��OH��2 |
| ��ʼ����pH | 4��7 | 2��7 | 7��6 |
| ��ȫ����pH | 6��7 | 3��7 | 9��6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����Fe��Cu��ɵĺϽ�����Cu��Fe�������ʵ���Ϊa mol��Cu�����ʵ�������Ϊx���гɷ�ĩ��ȫ��Ͷ�뺬b mol HNO ��ϡ��Һ�У���ʹ���ַ�Ӧ��������Ļ�ԭ����ֻ��NO���Իش��������⣺
��ϡ��Һ�У���ʹ���ַ�Ӧ��������Ļ�ԭ����ֻ��NO���Իش��������⣺
��1����ʣ��IJ���ֻ��Cu������Һ�к��еĽ�������Ϊ__________��(д�����п������)
��2������Һ�н�������ֻ��Fe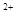 ��Cu
��Cu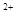 ʱ����b��ȡֵ��Χ��(��a��x��ʾ)___________��
ʱ����b��ȡֵ��Χ��(��a��x��ʾ)___________��
��3����x��0.5����Һ��Fe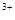 ��Fe
��Fe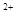 �����ʵ������ʱ���ڱ�״���¹�����672 mL���塣��a��____________��b��_____________��
�����ʵ������ʱ���ڱ�״���¹�����672 mL���塣��a��____________��b��_____________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com