
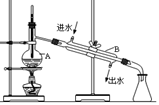


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
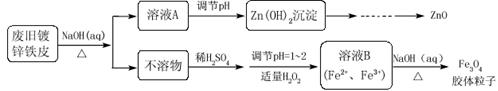
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A����25mL��ʽ�ζ�����ȡ20.00mLNaHCO3 |
| B����������ƽȷ����10.20��̼���ƹ��� |
| C����100mL��Ͳ��ȡ3.2mLŨ���� |
| D������1 mol��L�C1������������Һ475mLѡ��500mL����ƿ |
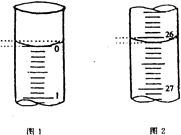
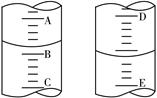
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Һ���ӷ����ڴ��Һ����Լ�ƿ��Ӧ��ˮ�� |
| B������������մ��Ƥ���ϣ������øɲ�������Ȼ��������������Һ��ϴ |
| C��ʵ��������һ�����ʵ���Ũ�ȵ���Һ������ʱ���ӿ̶��ߣ���������ҺŨ��ƫ�� |
| D��ij��Һ�м���BaC12��Һ�������˲�����ϡ����İ�ɫ����������Һ��һ������Ag+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| A��H2C2O4��2H2O | B��FeSO4 | C��Ũ���� | D��Na2SO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����Ǹ������������ȣ���Ҫ�ӵ�ʯ�������Է�����ը�� |
| B����ȡ������ˮ���ռ�����������Ӧ����ֹͣ���� |
| C��ŨNaOH��Һ����Ƥ���ϣ�������ˮ��ϴ��Ȼ��Ϳ��ϡ������Һ |
| D��Ũ���ὦ��Ƥ���ϣ�������ϡNaOH��Һϴ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
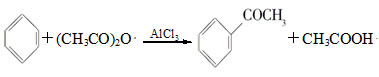
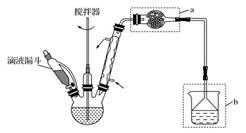
| A����Ӧ̫���� | B��Һ��̫������� | C����Ӧ�仺�� | D������������ |
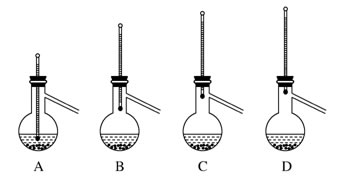
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Һʱ����Һ©���²�Һ����¿ڷų����ϲ�Һ����Ͽڵ����� |
| B������ʱ���¶ȼ�ˮ����Ӧ��û��Һ���С� |
| C�������У���ȴˮӦ�������ܵ��¿�ͨ�룬�Ͽ������� |
| D���Ѽ���FeCl3������Һ����250mL�ķ�ˮ����ȡFe(OH)3���塣 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ɫʯ����ֽ | B����ɫʯ����ֽ | C������KI��ֽ | D��pH��ֽ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com