
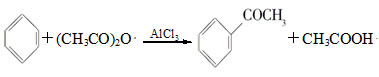
| A����Ӧ̫���� | B��Һ��̫������� | C����Ӧ�仺�� | D������������ |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| �ζ� ���� | ������Һ����� /mL | ����Һ�����/mL | |
| �ζ�ǰ�̶� | �ζ���̶� | ||
| 1 | 25��00 | 1��02 | 21��03 |
| 2 | 25��00 | 2��00 | 21��99 |
| 3 | 25��00 | 0��20 | 20��20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
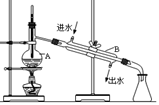
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | ʵ�����ģ�ʡ�Լг�װ�ã� | ��Ӧʵ�� |
| A | �Թܡ���ͷ�ι� | ��ϡ���ᡢNa2CO3��Һ�Ƚ�������̼�������ǿ�� |
| B | �ձ�������������ͷ�ιܡ���ֽ | �������ȥ���ᱵ�е�����̼�ᱵ |
| C | �ձ�������������ͷ�ιܡ�����ƿ | �ù����Ȼ�������0.5mol/L����Һ |
| D | �ձ����������������� | ����ͭ��Һ��Ũ���ᾧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| A����������ƽ����17.55g�Ȼ��ƾ��� |
| B��̼������Һ�����ڴ����������Լ�ƿ |
| C���ø����pH��ֽ�ⶨ������ˮ��pH |
| D��ʹ������ƿ������Һʱ�����ӿ̶��߶��ݺ�Ũ��ƫ�� |
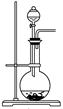
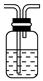
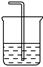
| ���� | O2 | Cl2 | NH3 |
| ��Һ©�����Լ� | | | Ũ��ˮ |
| Բ����ƿ���Լ� | | KMnO4 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
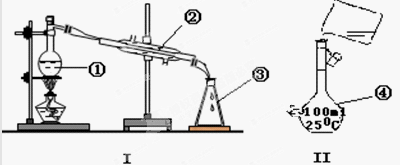
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

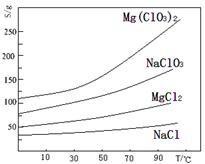
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
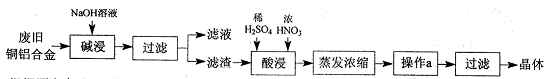
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com