
���������е�+4�����������ԣ����л�ԭ�ԡ������Լ�����ˮ��Na2S��Һ�� Na2SO3��Һ��ϡ���ᡢNaOH��Һ����ˮ��
(1)Ҫ֤��Na2SO3���л�ԭ�ԣ�Ӧѡ�õ��Լ��Уߣߣߣߣߣ������������ǣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��Ӧ�����ӷ���ʽΪ���ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
(2)Ҫ֤��Na2SO3���������ԣ�Ӧѡ�õ��Լ��Уߣߣߣߣߣ������������ǣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��Ӧ�����ӷ���ʽΪ���ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
 ������ÿ�ʱ��ҵϵ�д�
������ÿ�ʱ��ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ�Ͽ���ͭм��Ũ����Ϊԭ����ȡ����ͭ����ʵ�������У��Ȱ�ͭм�ڿ��������գ��ٸ��õ����ˮϡ�͵�Ũ���ᷴӦ����ȡ����ͭ����ش��������⣺
(1)��������ͭмֱ�������ᷴӦ����ȡ����ͭ��ԭ���� ��
(2)Ũ�����õ����ˮϡ�͵�Ŀ���� ��
(3)Ҫ�õ�����ͭ���壬Ӧѡ�� ��
(4)��Ӧ��������ֳ� �ԡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
һЩ������ѧ��Ӧ������ʽ��ʾ A + B �� C+D+H2O ����AΪ���� (��Ҫʱ�ɼ���)����������������ʾ�ش��������⣺
��1����A��C��D��������Ԫ�أ���A��B��Һ��Ӧ�����ӷ���ʽΪ�� ��
��2����C��D��Ϊ��������һ��Ϊ����ɫ����B�� ��
��3����C��D��Ϊ�����Ҷ���ʹ����ʯ��ˮ����ǣ���A��B��Ӧ�Ļ�ѧ����ʽΪ�� ��
���ϡ��B��Ũ��Һ ��
(4) ��AΪ�Ϻ�ɫ�Ĺ��壬DΪ��ɫ��ζ�����壬��A��B��Һ��Ӧ�����ӷ���ʽΪ�� ����������״����4��48L��D���壬��ԭ��B�����ʵ����� mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������һ����Ҫ�������ܲ���,ij�о���ѧϰС����Ƶ����ú��ߵ�ʯӢɰ(���Ȼ��ơ�������������)�Ʊ������Ƶ���������: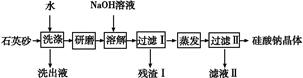
(1)ʯӢɰ��ˮϴ�ӵ�Ŀ���� ��
��ĥ�����NaOH��Һ�ܽ�����ӷ���ʽ�� ����
(2)ʵ���ҽ�����������Ҫ�õ�������������̨(����Ȧ)����������������������
(3)������ijɷ�����������(�ѧʽ)������������������,�ټ���NaOH��Һ�õ�����,���ó������뵽NaClO��NaOH�����Һ�п��Ƶ�һ�����ʾ�ˮ��,��ɷ�Ӧ�����ӷ���ʽ:���� ������+��ClO-+��OH- ���� ������+��Cl-+��H2O
���� ������+��Cl-+��H2O
(4)�����������ƵõĹ����ƾ���ɱ�ʾΪNa2O��nSiO2,��ʯӢɰ������Ϊ10.0 g,���к�SiO2����������Ϊ90%,���յõ������ƾ���15.2 g,��n=����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
±�ص��ʵ����ʻ��ã�±�صĻ�����Ӧ�ù㷺�����û�ѧ��Ӧԭ���о�±��Ԫ�ص��й����ʾ�����Ҫ���塣
��1�����й�����ˮ��������ȷ����_______����д��ţ���
A����ˮ�д������ֵ���ƽ��
B������ˮ��ͨ��SO2����Ư������ǿ
C������ˮ��ͨ��������c( H��)/c(ClO��)��С
D����ˮϡ����ˮ����Һ�е���������Ũ�Ⱦ���С
E����ˮϡ����ˮ��ˮ�ĵ���ƽ��������Ӧ�����ƶ�
F������ˮ�м���������NaOH��������c(Na��)=c(Cl�� )+c(ClO��)
��2����ҵ��ͨ���ȼҵ�����������䷴Ӧ�����ӷ���ʽΪ______��
��3�������£���֪25��ʱ�й�����ĵ���ƽ�ⳣ����
д��84����Һ����Ҫ�ɷ�ΪNaClO��¶���ڿ����з�����Ӧ���йػ�ѧ����ʽ________������84����Һ����������Ũ���ᣩ���ʹ�ÿ��ܻᵼ���ж����������ӷ���ʽ�����й�ԭ��___________��
��4�����ٵƾ��бȰ׳���������һ������ܵģ��ص㡣һ���¶��£������ڷ�����������ʹ�ù����г����ڹܱ��ϵ��ٿ��Է�����Ӧ��  ��Ϊģ��������Ӧ��ȷ��ȡ0. 508g�⡢0.736g����������50. 0mL���ܱ������У�����ʹ�䷴Ӧ����ͼ�� WI2(g)�����ʵ�����ʱ��仯��ϵͼ����������I��0��t2ʱ��Σ��ķ�Ӧ�¶�ΪT1������II(��t2��ʼ)�ķ�Ӧ�¶�ΪT2����T2>T1����
��Ϊģ��������Ӧ��ȷ��ȡ0. 508g�⡢0.736g����������50. 0mL���ܱ������У�����ʹ�䷴Ӧ����ͼ�� WI2(g)�����ʵ�����ʱ��仯��ϵͼ����������I��0��t2ʱ��Σ��ķ�Ӧ�¶�ΪT1������II(��t2��ʼ)�ķ�Ӧ�¶�ΪT2����T2>T1����
�ٸ÷�Ӧ�ġ�H_______0���>����=��<����
�ڴӷ�Ӧ��ʼ��t1ʱ���ڵ�ƽ����Ӧ����v(I2)=_________��
������˵���в���ȷ����_________������ţ���
A�����ø÷�Ӧԭ�������ᴿ��
B���÷�Ӧ��ƽ�ⳣ������ʽ��K=
C����˿�����¶�Խ�ߣ���˿��������WI2Խ�ױ�ΪW�����³�������˿��
��5��25��ʱ����5mL����KCI��KIŨ�Ⱦ�Ϊ0.1mol/L�Ļ��Һ�У��μ�6mL0��1mol/L��AgNO3��Һ�������ɵij�����_________����Һ������Ũ���ɴ�С��˳����_______ [������H����OH����25��ʱ
 ]��
]��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijͬѧ������ͼװ����NO���������ʵ��̽����
��1��д���Ʊ�NO�ķ�Ӧ����ʽ
��2���ռ��������������Ϻ���ɫ����˵��NO����_____�ԡ�
��3���Ʊ�NO�IJ������£�
1��ͼ��ʾ���Ӻ�װ�ã�
2ȡ��ע������ͨ��U�ͳ��ܼ���ϡ�������ӽ�U�Ͷ̹ܵ�ͭ˿
3����ͷ�ϰ�װ��ע���������U�Ͷ̹��е����п�����Ȼ��γ�ע���������ž�ע�����еĿ�����
4��������ͭ˿����������ų�������ע�����������NO����ע������ͷ������Ƥ�����Է�ڡ�
��������������©��һ�������� ���ڲ���3�п��Թ۲쵽������Ϊ ��
��4����ͭ˿�ϰ���ʯī�������� ��
��5������ϡ���ỻ��Ũ���ᣬ�����ȡNO2���塣������ע�����ռ�NO2���岢��ɡ�������ضԻ�ѧƽ���ƶ�Ӱ�족ʵ��̽����������������������ֻ���һ��Ӱ�����ص�̽��������ʵ����Ʒ��ѡ����֪
2NO2(g)  N2O4(g) ��H ��0��N2O4Ϊ��ɫ���塣
N2O4(g) ��H ��0��N2O4Ϊ��ɫ���塣
| ʵ�鲽�衡�� | ʵ������ | ʵ����ۡ��� |
| �ٷֱ�����֧ע�����ռ�NO2������ע������ͷ������Ƥ�����Է�ڡ����� ��_____________________���� _______________________���� ________________________���� _______________________���� ________________________���� | _______________________���� ________________________���� _______________________���� ________________________���� _______________________���� _______________________���� ________________________���� _______________________���� | _______________________���� ________________________���� _______________________���� ________________________���� _______________________���� _______________________���� ________________________���� _______________________���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
AԪ�ص�һ�ֵ�����һ����Ҫ�İ뵼����ϣ���AԪ�ص�һ�ֻ�����C��������������ܵ��ִ�ͨѶ���ϡ������ά��C���ռӦ���ɺ�AԪ�صĻ�����D��
��1����Ԫ�����ڱ��У�Aλ�ڵ�________�壬��Aͬ�嵫���ԭ��������AС��Ԫ��B��ԭ�ӽṹʾ��ͼΪ________��A��B��ԭ�ӵĵ��Ӳ�ṹ�ϵ���ͬ����_______________________��
��2������C������ѧ��Ӧ������______ �������ƣ�����Ӧ�Ļ�ѧ����ʽ��_______��
��3����C�봿���ϸ�������ʱҲ������ѧ��Ӧ����D��ͬʱ������B�����������E����ȫ����E��ȫ����D��������ˮ�л�Ϻ��ַ�����ѧ��Ӧ���ɺ�A�Ļ�����F��
��д������D��F�Ļ�ѧ����ʽ��______________________________________��___________________________________________________________________��
��Ҫ��NaOH�����ۻ������������в���ѡ�õ���_________________________��
A.��ͨ�������� B.ʯӢ��������
C.���������� D.������
��4��100 g C��ʯ��ʯ�Ļ�����ַ�Ӧ�����ɵ������ڱ�״���µ����Ϊ11.2 L��100 g�������ʯ��ʯ������������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ˮ���ж������ʣ�����������ˮ�ֱ�����ͼ�������ʷ����ķ�Ӧ���(��ˮ����)��
��1��a��b��c�з�Ӧ�����ӷ���ʽΪ�� ��
e�еĻ�ѧ��Ӧ����ʽΪ ��������Ӧ�з�����������ԭ��Ӧ���ǣ� (�a������b������c����e��)��
��2����֤����ˮ����Ư���Ե�������____________________________��
��3�����õ���ˮ��Ϊ________���û�ѧ��Ӧ����ʽ��ʾΪ__________��
��4��ʵ���ұ��汥����ˮ�ķ�����_____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ���������β���к��е�������NOx��NO��NO2�Ļ������費��N2O4��������̬���������ཡ�������ϴ����в��
��1����ҵ�Ͽ��ð������շ�����NOx����Ӧԭ��Ϊ��
 ��ij��ѧ��ȤС��ģ��ô������̵�ʵ��װ�����£��г�װ������ȥ����
��ij��ѧ��ȤС��ģ��ô������̵�ʵ��װ�����£��г�װ������ȥ����
��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��װ��D�м�ʯ�ҵ������� ��
��2����ҵ��Ҳ����Na2CO3��Һ���շ�����NOx����֪��NO������Na2CO3��Һ��Ӧ��
NO+ NO2+Na2CO3=2NaNO2+ CO2��2NO2+Na2CO3= NaNO2+ NaNO3+CO2��
�ٵ�NOx��Na2CO3��Һ��ȫ����ʱ��x��ֵ�������� ������ĸ����
A��1.3 B��1.6 C��1.8
�ڽ�1 mol NOxͨ��Na2CO3��Һ�У�����ȫ����ʱ����Һ�����ɵ�NO��3��NO��2�������ӵ����ʵ�����x�仯��ϵ��ͼ��ʾ��
ͼ���߶�a��ʾ ������xֵ�仯�Ĺ�ϵ������������������Ϊ21.2%��Na2CO3��Һ���գ�����ҪNa2CO3��Һ���� g��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com