
����Ŀ���ڵ����仯����Ļ��������У����йط�Ӧ�ķ�Ӧԭ�������о�������Ҫ���塣
��1��t��ʱ������N2��NH3��������Ӧ����Ϣ���±���ʾ��
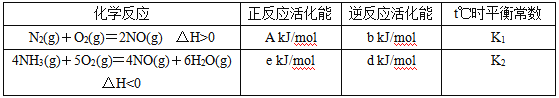
��д��t��ʱ������һ����������������������Ȼ�ѧ����ʽ��______________________��t��ʱ�÷�Ӧ��ƽ�ⳣ��Ϊ__________ (��K1��K2��ʾ)��
��2����ҵ�ϳɰ���ԭ��Ϊ��N2(g)��3H2(g)![]() 2NH3(g)��ͼ�ױ�ʾ��һ��������ܱ������з�Ӧʱ��H2�����ʵ���Ũ����ʱ��ı仯��ͼ�ұ�ʾ�������������������£���ʼͶ��H2��N2�����ʵ���֮��(��Ϊx)��ƽ��ʱNH3�����ʵ��������Ĺ�ϵ��
2NH3(g)��ͼ�ױ�ʾ��һ��������ܱ������з�Ӧʱ��H2�����ʵ���Ũ����ʱ��ı仯��ͼ�ұ�ʾ�������������������£���ʼͶ��H2��N2�����ʵ���֮��(��Ϊx)��ƽ��ʱNH3�����ʵ��������Ĺ�ϵ��
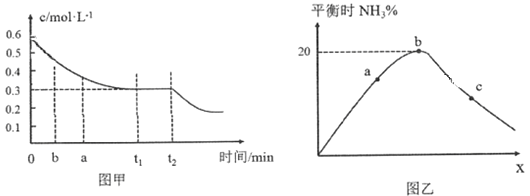
��ͼ����0��t1 min�ڣ�v(N2)��_____mol��L��1��min��1��b���v(H2)��_____a���v(H2)��(������������С��������������)��
��ͼ���У�b��ʱN2�����ʵ�������__________��
����֪ij�¶��¸÷�Ӧ��ƽ��ʱ�����ʾ�Ϊ1 mol���������Ϊ1L�������¶Ⱥ�ѹǿ���䣬�ֳ���3 mol N2��ƽ��________(���������ƶ����������ƶ����������ƶ���)��
���𰸡�4NH3(g)+6NO(g)=5N2(g)+6H2O(g) ��H=[(e-d)-5(A-b)] kJmol-1 ![]()
![]() molL-1min-1 ���� 20% ���ƶ�
molL-1min-1 ���� 20% ���ƶ�
��������
(1)���ݱ������ݣ���Ӧ����H=����Ӧ�Ļ��-�淴Ӧ�Ļ�ܼ������Ӧ�ٺ͢ڵ���H���ٸ��ݸ�˹���ɷ������
(2)�ٸ���ͼ��0��t1 min�ڣ���c(H2)=(0.6-0.3)molL-1=0.3molL-1������c (N2)=0.1molL-1���ݴ˼���v(N2)����H2��N2����ʼ����ȷ��Ϸ���ʽ�л�ѧ������֮��ʱ���ﵽƽ��ʱ��������������ͼ���У�b��ʱx=3��1����a��xС��3��1���ݴ˷����жϣ�
�ڵ�H2��N2����ʼ����ȷ��Ϸ���ʽ�л�ѧ������֮��ʱ���ﵽƽ��ʱ��������������ͼ���У�b��ʱx=3��1��ƽ��ʱ���������ʵ�������Ϊ20%����������ʽ�������㣻
�ۼ�֪ij�¶��¸÷�Ӧ��ƽ��ʱ�����ʾ�Ϊ1 mol���������Ϊ1L���������ʱ��ƽ�ⳣ��K�������¶Ⱥ�ѹǿ���䣬�ֳ���3 mol N2��������������2L�������Qc������K�Ƚ��жϡ�
(1)���ݱ������ݣ���Ӧ����H=����Ӧ�Ļ��-�淴Ӧ�Ļ�ܣ���N2(g)+O2(g)=2NO(g) ��H=(A-b) kJmol-1����4NH3(g)+5O2(g)=4NO(g)+6H2O(g) ��H=(e-d) kJmol-1�����ݸ�˹���ɣ�����-����5�õ�������һ����������������������Ȼ�ѧ����ʽ��4NH3(g)+6NO(g)=5N2(g)+6H2O(g) ��H=[(e-d)-(A-b)��5]kJmol-1=[(e-d)-5(A-b)]kJmol-1��t��ʱ�÷�Ӧ��ƽ�ⳣ��K=![]() ���ʴ�Ϊ��4NH3(g)+6NO(g)=5N2(g)+6H2O(g)��H=[(e-d)-5(A-b)] kJmol-1��
���ʴ�Ϊ��4NH3(g)+6NO(g)=5N2(g)+6H2O(g)��H=[(e-d)-5(A-b)] kJmol-1��![]() ��
��
(2)��0��t1 min�ڣ���c(H2)=(0.6-0.3)molL-1=0.3molL-1������c (N2)=0.1molL-1����v(N2)=![]() =
=![]() =
=![]() molL-1min-1����H2��N2����ʼ����ȷ��Ϸ���ʽ�л�ѧ������֮��ʱ���ﵽƽ��ʱ��������������ͼ���У�b��ʱx=3��1����a��xС��3��1�������������䣬��ƽ��ʱ������Ũ��С��b����b���v(H2)����a���v(H2)�����ʴ�Ϊ��
molL-1min-1����H2��N2����ʼ����ȷ��Ϸ���ʽ�л�ѧ������֮��ʱ���ﵽƽ��ʱ��������������ͼ���У�b��ʱx=3��1����a��xС��3��1�������������䣬��ƽ��ʱ������Ũ��С��b����b���v(H2)����a���v(H2)�����ʴ�Ϊ��![]() molL-1min-1�����ڣ�
molL-1min-1�����ڣ�
�ڵ�H2��N2����ʼ����ȷ��Ϸ���ʽ�л�ѧ������֮��ʱ���ﵽƽ��ʱ��������������ͼ���У�b��ʱx=3��1��ƽ��ʱ���������ʵ�������Ϊ20%������ʼʱH2��N2�����ʵ����ֱ�Ϊ3mol��1mol�����ɵİ���Ϊ2xmol
N2(g)��3H2(g) 2NH3(g)
��ʼ(mol) 1 3 0
��Ӧ(mol) x 3x 2x
ƽ��(mol)1-x 3-3x 2x
��![]() ��100%=20%�����x=
��100%=20%�����x=![]() ��b��ʱN2�����ʵ�������=
��b��ʱN2�����ʵ�������=![]() =20%���ʴ�Ϊ��20%��
=20%���ʴ�Ϊ��20%��
�ۼ�֪ij�¶��¸÷�Ӧ��ƽ��ʱ�����ʾ�Ϊ1 mol���������Ϊ1L����ʱ��ƽ�ⳣ��K=![]() =1�������¶Ⱥ�ѹǿ���䣬�ֳ���3 mol N2��������������2L��Qc=
=1�������¶Ⱥ�ѹǿ���䣬�ֳ���3 mol N2��������������2L��Qc=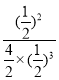 =1=K��Ϊƽ��״̬��ƽ�ⲻ�ƶ����ʴ�Ϊ�����ƶ���
=1=K��Ϊƽ��״̬��ƽ�ⲻ�ƶ����ʴ�Ϊ�����ƶ���


 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������߷ֱ��ʾԪ�ص�ij��������˵�����Ĺ�ϵ(ZΪ�˵������YΪԪ�ص��й�����)��
(1)��������Ԫ���й�������������߱��������Ӧ�Ŀո���:
a. 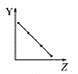 b.
b. 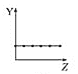 c.
c. 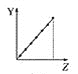 d.
d. 
�ٵڢ�A��Ԫ�صļ۵�����________��
�ڵ�������Ԫ�ص�����ϼ�________��
��F-��Na+��Mg2+��Al3+�����Ӱ뾶________��
(2)Ԫ��X��Y��Z��M��N��Ϊ����������Ԫ�أ���ԭ����������������֪Yԭ������������������������֮��Ϊ3��4��MԪ��ԭ�ӵ���������������Ӳ���֮��Ϊ4��3��N-��Z+��X+�İ뾶��С��������XN������Ϊ���塣�ݴ˻ش�
��XΪ___________(����)��YΪ____________(Ԫ�ط���)��Zԭ�ӽṹʾ��ͼΪ________________��
��N������������ˮ����Ļ�ѧʽΪ________________��
��M�����������Ļ�ѧʽΪ________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ɫ��ѧ��ָ�Ӽ�������������ƿ��еĻ�ѧ��Ӧ�������ܼ��ٶԻ����ĸ����á����� ��ѧ��Ӧ��������ɫ��ѧ�������( )
A. �������᳧β���ŷţ�SO2+2NH3+H2O=(NH4)2SO3
B. ���������᳧�ĵ���������Ⱦ��NO2+NO+2NaOH=2NaNO2+H2O
C. �� CuSO4��Cu+2H2SO4(Ũ) ![]() CuSO4+SO2��+2H2O
CuSO4+SO2��+2H2O
D. �� CuSO4��2Cu+O2![]() 2CuO��CuO+H2SO4(ϡ)=CuSO4+H2O
2CuO��CuO+H2SO4(ϡ)=CuSO4+H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ʵ�������ö������̸�Ũ���ᷴӦ�Ʊ����﴿�������������д�ʵ�飬����������ͼ��ʾ��
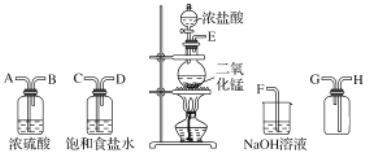
��1������������������ȷ˳������ӿڴ�����ĸ����____ ��____��____��____��____��_____��____��____��
��2��װ���У�����ʳ��ˮ��������________��NaOH��Һ��������_______��
��3����ѧʵ���г���ʪ��ĵ��ۣ�KI��ֽ�����Ƿ���Cl2�����������Cl2�������ɹ۲쵽_____����Ӧ�Ļ�ѧ����ʽΪ________��
��4��д�����з�Ӧ�����ӷ���ʽ��
�����巢��װ���н��еķ�Ӧ��____��
��NaOH��Һ�з����ķ�Ӧ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧʵ���Ҳ����ķ�Һ�к��д�������Ⱦ���������ʣ�Ϊ�˱�����������ѧ�����Ҳ����ķ�Һ���뾭����������ŷš�ij��ѧʵ���Ҳ��������Է�Һ�к���Fe3+��Cu2+��Ba2+���ֽ��������Ӻ�Cl-�������ӣ�ʵ������������������Է�Һ���д������Ի��ս������ⶨ����������������
��֪ʵ���д��������Է�Һ�����ΪIL����pH�Ʋⶨ������H+���ʵ���Ũ��Ϊ0.10mol��L-1.
��ش��������⣺.
(1)ʵ���в���I��II��III�ķ�����ͬ��������������Ϊ���ˡ�_________�����
(2)������������������Һ��ɫΪ�ػ�ɫ���÷�Ӧ�����ӷ���ʽΪ��_________
(3)������ŷŵķ�ˮ�в���Fe3+�ķ����ǣ�_________
(4)ʵ���г�������I������Ϊ4.66g������II������Ϊ15.2g����������ϡ�����ܽ����II������˱�״���µ���ɫ����4.48L�������II�н���ͭ������Ϊ_________
(5)ʵ���н�����II���г�����գ�ʹ���������˾ƾ��ơ����żܡ������ǡ��������⣬����_________(������)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ���ǣ� ��
A. CH3(C2H5)CHCH(CH3)2��ϵͳ����Ϊ2������3���һ�����
B. ������Ľṹ��ʽΪ![]()
C. �����Ҵ��Ļ�ԭ���Լ�Cr3����Cr2O72������ɫ����������ƺ�ݳ�
D. C5H10��ͬ���칹��������ϩ������6��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й��ڹż��еļ���˵������ȷ����
A. �����ݸ�Ŀ�����վ�����Ŀ��д����Ԫʱʼ���䷨����Ũ�ƺ�����굣���������������ˮ��ζ��Ũ�ң��Ǿ�¶Ҳ�����������õ���������ָ����
B. �����ϴ���������ࡷ������(��ͭ)�����ᣬ����������� �����˺Ͻ�Ӳ�ȷ��������
C. �����ݾ���ע���й��ڼ�����ʯ(KNO3)������(Na2SO4)�ļ��أ����Ի���֮����������������ʯҲ�����÷���Ӧ������ɫ��Ӧ
D. ������������ƪ���м��أ�����ɰ(HgS)��֮��ˮ���������ֳɵ�ɰ�����ù��̷����˷ֽ⡢���ϡ�������ԭ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������������Ķ��ˮ��������K2FeO4Ϊ��ɫ���壬������ˮ������ŨKOH��Һ���������л����0��5�桢ǿ������Һ�бȽ��ȶ��������ԡ�������Һ���ֽ�ų�O2��ijʵ��С���Ʊ�������أ�K2FeO4�����ⶨ��Ʒ���ȡ��ش��������⣺
��.�Ʊ�K2FeO4
װ������ͼ��ʾ���г֡����ȵ�װ���ԡ�
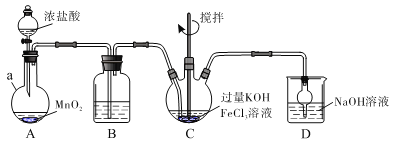
(1)����a��������____________��װ��B�г����������Լ���__________��װ��D��������________________��
(2)A�з�����Ӧ�Ļ�ѧ����ʽΪ___________________________________��
(3)C�з�ӦΪ���ȷ�Ӧ������Ӧ�¶��������0��5�棬���õĿ��·���Ϊ______����Ӧ��KOH���������ԭ����_____________________��
(4)д��C���ܷ�Ӧ�����ӷ���ʽ��__________________��C�л���ᆳ�ؽᾧ���л���ϴ�Ӵ�������ո���õ�������ؾ��塣
��.K2FeO4��Ʒ���ȵIJⶨ
ȷ��ȡ1.00g�Ƶõľ��壬���250mL��Һ��ȷ��ȡ25.00mL K2FeO4��Һ������ƿ����������CrCl3��NaOH��Һ�����ټ���ϡ�����ữ���Fe3+��Cr2O![]() �����뼸�ζ�������������ָʾ������0.0500mol/L (NH4)2Fe(SO4)2����Һ�ζ����յ㣨��Һ��dz�Ϻ�ɫ����ƽ�вⶨ���Σ�ƽ������ (NH4)2Fe(SO4)2����Һ28.00mL��
�����뼸�ζ�������������ָʾ������0.0500mol/L (NH4)2Fe(SO4)2����Һ�ζ����յ㣨��Һ��dz�Ϻ�ɫ����ƽ�вⶨ���Σ�ƽ������ (NH4)2Fe(SO4)2����Һ28.00mL��
(5)�����������ݣ���Ʒ��K2FeO4����������Ϊ__________����(NH4)2Fe(SO4)2����Һ���ֱ��ʣ���ʹ�ⶨ���_______������ƫ������ƫ����������Ӱ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����18.0 g��Cu��Al��Fe��ɵĺϽ�����������NaOH��Һ�У��Ͻ�����������5.4 g����ȡ�������ĺϽ����ڹ���ϡHNO3�У�������8.96 L NO(��״����)����Ӧ�����Һ�м��������NaOH��Һ�������������Ϊ
A.22.8 gB.25.4 gC.33.2gD.������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com