
����˵������ȷ����
A����֪�����ۻ���Ϊ6.0 kJ/mol�������������Ϊ20 kJ/mol������1 mol������2 mol ��������ۻ�����ȫ�����ƻ���������������ֻ���ƻ�����15�������
B����֪һ���¶��£�������Һ�����ʵ���Ũ��Ϊc�������Ϊ����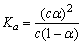 �����������������ƹ��壬��CH3COOH
�����������������ƹ��壬��CH3COOH CH3COO����H�������ƶ�������С��Ka��С
CH3COO����H�������ƶ�������С��Ka��С
C��ʵ���û�����(l)������ϩ(l)�ͱ�(l)�ı�ȼ���ȷֱ�Ϊ��3916 kJ/mol����3747 kJ/mo l�ͣ�3265 kJ/mol������֤���ڱ������в����ڶ�����̼̼˫��
l�ͣ�3265 kJ/mol������֤���ڱ������в����ڶ�����̼̼˫��
D����֪��Fe2O3(s)��3C(ʯī) 2Fe(s)��3CO(g)����H����489.0 kJ/mol��
2Fe(s)��3CO(g)����H����489.0 kJ/mol��
CO(g)��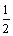 O2(g)
O2(g) CO2(g)����H����283.0 kJ/mol��
CO2(g)����H����283.0 kJ/mol��
C(ʯī)��O2(g) CO2(g)����H����393.5 kJ/mol��
CO2(g)����H����393.5 kJ/mol��
��4Fe(s)��3O2(g) 2Fe2O3(s)����H����1641.0 kJ/mol
2Fe2O3(s)����H����1641.0 kJ/mol
��������A����ȷ���ۻ���ֻ�൱��0.3 mol�����B������Kaֻ���¶��йأ���Ũ���أ� C����ȷ������ϩ(l)�뻷����(l)��ȣ��γ�һ��˫������������169kJ/mol����(l)�뻷����(l)��ȣ���������691kJ/mol��Զ����169��3��˵�������������ȶ��ṹ��D����ȷ���ȷ���ʽ�٣�(�ۣ���)��3���ܡ�2����HҲ������
C����ȷ������ϩ(l)�뻷����(l)��ȣ��γ�һ��˫������������169kJ/mol����(l)�뻷����(l)��ȣ���������691kJ/mol��Զ����169��3��˵�������������ȶ��ṹ��D����ȷ���ȷ���ʽ�٣�(�ۣ���)��3���ܡ�2����HҲ������
������������Ϊ���ۺ��⣬��Ҫ���������ʵļ��ܷ���Ӧ�� ����ѧ��Ӧ�����仯�ĸ�˹���ɵ�Ӧ�ã��Լ����������Һ�ĵ���ƽ�������
����ѧ��Ӧ�����仯�ĸ�˹���ɵ�Ӧ�ã��Լ����������Һ�ĵ���ƽ�������
���𰸡�B



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��C(s)��H2O(g)===CO(g)��H2(g)����H��a kJ��mol��1
2C(s)��O2(g)===2CO(g)����H����220 kJ��mol��1
H��H��OO��OH���ļ��ֱܷ�Ϊ436 kJ��mol��1��496 kJ��mol��1��462 kJ��mol��1����aΪ(����)
A����332 B����118
C����350 D����130
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��FeCl3������Һ�ѳ�H2S��ķ�Һ��ͨ�����Ƶ�ѹ������������ijͬѧʹ��ʯī�缫���ڲ�ͬ��ѹ(x)�µ��pH��1��0.1 mol/L FeCl2��Һ���о���Һ������������¼����(a��b��c������ѹֵ)��
| ��� | ��ѹ/V | �������� | ������������ |
| �� | x��a | �缫�������ֻ�ɫ�������ݲ��� | ��Fe3������Cl2 |
| �� | a��x��b | �缫�������ֻ�ɫ�������ݲ��� | ��Fe3������Cl2 |
| �� | b��x��0 | �����Ա仯 | ��Fe3������Cl2 |
(1)��KSCN��Һ�����Fe3����������________��
(2)���У�Fe3��������ԭ������Cl���������ŵ磬���ɵ�Cl2��Fe2��������д���йط�Ӧ�����ӷ���ʽ��________________________________________________��
(3)�ɢ��Ʋ⣬Fe3��������ԭ������Fe2���������ŵ硣ԭ����Fe2������________�ԡ�
(4)������δ�����Cl2����Cl���������Ƿ�ŵ������һ����֤�����pH��1��NaCl��Һ������ʵ�飬��¼���£�
| ��� | ��ѹ/V | �������� | ������������ |
| �� | a��x��c | �����Ա仯 | ��Cl2 |
| �� | c��x��b | �����Ա仯 | ��Cl2 |
��NaCl��Һ��Ũ����________mol/L��
�ڢ��м���Cl2��ʵ�鷽����_____________________________________________________________________��
�����Աȣ��ó��Ľ���(д������)��______________________________________________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й�˵����ȷ����
A��CaCO3(s)=CaO(s)��CO2(g)�����²����Է����У�˵���÷�Ӧ�ġ�H��0
B����ͭ����Ʒ�Ʋ����������Ʒ������ǰ����������
C��N2(g)��3H2(g) 2NH3(g) ��H��0��������������ʱ�����¶ȣ���Ӧ���ʣ�(H2)��������ƽ��ת���ʾ�����
2NH3(g) ��H��0��������������ʱ�����¶ȣ���Ӧ���ʣ�(H2)��������ƽ��ת���ʾ�����
D��ˮ�����ӻ�����Kw�����¶ȵ����߶�����˵��ˮ�ĵ����Ƿ��ȷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Cl2����ijЩ�����л���ʱ�����������HC1�����÷�ӦA����ʵ���ȵ�ѭ�����á���ӦA��4HCl��O2
 2Cl2��2H2O
2Cl2��2H2O
(1)��֪:��ӦA�У� 4mol HCI���������ų�115.6kJ��������
��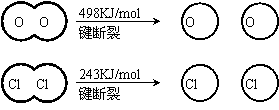

H2O�ĵ���ʽ��_______________.
�ڷ�ӦA���Ȼ�ѧ����ʽ��_______________��
�۶Ͽ�1 mol H��O ����Ͽ� 1 mol H��Cl �������������ԼΪ__________KJ��H2O��H��O ����HCl��H��Cl�����ǿ����������_______________��
(2)���ڷ�ӦA����ͼ��4��Ͷ�ϱ�[n(HCl)��n(O2)���ֱ�Ϊ1��1��2��1��4��1��6��1��]�£���Ӧ�¶ȶ�HClƽ��ת����Ӱ������ߡ�
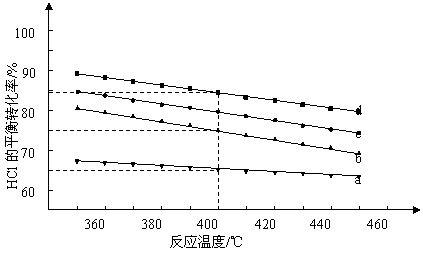

������b��Ӧ��Ͷ�ϱ���______________.
�ڵ�����b, c, d��Ӧ��Ͷ�ϱȴﵽ��ͬ��HClƽ��ת����ʱ����Ӧ�ķ�Ӧ�¶���Ͷ
�ϱȵĹ�ϵ��_________________.
��Ͷ�ϱ�Ϊ2:1���¶�Ϊ400��ʱ��ƽ��������Cl2�����ʵ���������_______________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
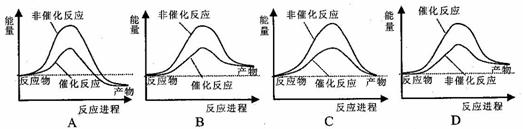
�ݱ�������ѧ�ҿ�����������̫���ֽܷ�ˮ�����ʹ����������й�ˮ�ֽ���̵������仯ʾ��ͼ��ȷ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����Ȼ�ѧ����ʽ�����ӷ���ʽ�У���ȷ���ǣ�
A.����ı�ȼ����Ϊ-890.3kJ��mol-1�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ��
CH4(g)+2O2(g)=CO2(g)+2H2O(g) ��H=-890.3kJ��mol-1
B. 500�桢30MPa�£���0.5mol N2��1.5molH2�����ܱյ������г�ַ�Ӧ����NH3(g)������19.3kJ�����Ȼ�ѧ����ʽΪ��
 ��H=-38.6kJ��
��H=-38.6kJ�� mol-1
mol-1
C. �Ȼ�þ��Һ�백ˮ��Ӧ��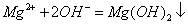
D. ����������NaOH��Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijѧ������ʵ��������з�����ϴ����������
����Ũ��ˮ��ϴ����������Ӧ����Թ�
���þƾ���ϴ�������������ձ�
����Ũ������ϴ����������طֽ�ʵ����Թ�
����������ϴ���ڴ�����Ȼ�����Һ���Լ�ƿ
��������������Һ��ϴʢ�����ӵ��Թ�
�������������Ƶ�Ũ��Һ��ϴմ����ǵ��Թ�
���ж����ϲ������жϡ���ȷ���� �� ��
A�������ⶼ�� B�������ⶼ�� C���ܢݲ��� D��ȫ����ȷ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵������ȷ���� �� ��
A��NaCl��Һ���磬����NaCl��Һ�ǵ���ʣ�
B��Cu�ܵ��磬����Cu�ǵ���ʣ�
C��SO3����ˮ�ܵ��磬����SO3�ǵ���ʣ�
D��Һ̬�ƾ����ƾ���ˮ��Һ�����磬���Ծƾ��Ƿǵ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com