
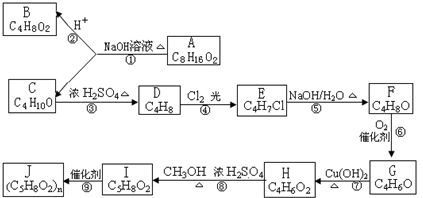
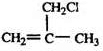 ��E����ˮ��õ���FΪCH2=C��CH3��CH2OH��F�����õ�GΪCH2=C��CH3��-CHO��������Ӧ�ߵõ�����HΪCH2=C��CH3��-COOH��H��CH3OH�õ���IΪCH2=C��CH3��-COOCH3����JΪ�Ӿ۷�Ӧ�IJ��Ϊ
��E����ˮ��õ���FΪCH2=C��CH3��CH2OH��F�����õ�GΪCH2=C��CH3��-CHO��������Ӧ�ߵõ�����HΪCH2=C��CH3��-COOH��H��CH3OH�õ���IΪCH2=C��CH3��-COOCH3����JΪ�Ӿ۷�Ӧ�IJ��Ϊ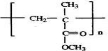 ���ݴ˴��⣻
���ݴ˴��⣻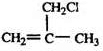 ��E����ˮ��õ���FΪCH2=C��CH3��CH2OH��F�����õ�GΪCH2=C��CH3��-CHO��������Ӧ�ߵõ�����HΪCH2=C��CH3��-COOH��H��CH3OH�õ���IΪCH2=C��CH3��-COOCH3����JΪ�Ӿ۷�Ӧ�IJ��Ϊ
��E����ˮ��õ���FΪCH2=C��CH3��CH2OH��F�����õ�GΪCH2=C��CH3��-CHO��������Ӧ�ߵõ�����HΪCH2=C��CH3��-COOH��H��CH3OH�õ���IΪCH2=C��CH3��-COOCH3����JΪ�Ӿ۷�Ӧ�IJ��Ϊ ��
��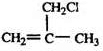 ��ˮ�ⷴӦ����Ӧ�ķ���ʽΪ
��ˮ�ⷴӦ����Ӧ�ķ���ʽΪ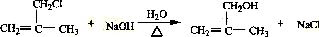 ����Ӧ��ΪCH2=C��CH3��CH2OH�����õ�CH2=C��CH3��-CHO����Ӧ�Ļ�ѧ��Ӧ����ʽΪ2CH2=C��CH3��CH2OH+O2
����Ӧ��ΪCH2=C��CH3��CH2OH�����õ�CH2=C��CH3��-CHO����Ӧ�Ļ�ѧ��Ӧ����ʽΪ2CH2=C��CH3��CH2OH+O2| ���� |
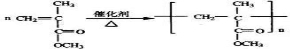 ��
��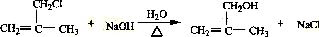 ��2CH2=C��CH3��CH2OH+O2
��2CH2=C��CH3��CH2OH+O2| ���� |
 ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������ʱΪ�ӿ��ٶȿ����ò����������� |
| B������ʱ���������ʯ������ |
| C�����þƾ���ȡ��ˮ�еĵ� |
| D������ʱ�¶ȼ�Һ��Ӧ��������ƿ֧�ܿ�ƽ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
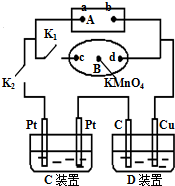 ��1����ͼ��ʾ��AΪ��Դ��BΪ������ʳ��ˮ�ͷ�̪��Һ����ֽ����ֽ�������һ��KMnO4��Һ��C��DΪ���ۣ���缫���ϼ�ͼ���������Һ����֪��
��1����ͼ��ʾ��AΪ��Դ��BΪ������ʳ��ˮ�ͷ�̪��Һ����ֽ����ֽ�������һ��KMnO4��Һ��C��DΪ���ۣ���缫���ϼ�ͼ���������Һ����֪���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���٢� | B���٢� | C���ڢ� | D���ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���٢� | B���ڢ� | C���ڢ� | D���٢� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������ͨ������������Һ�У�[Na+]=[Cl-]+[ClO-]+[OH-] |
| B��pH=8.3��NaHCO3��Һ��[Na+]��[HCO3-]��[CO32-]��[H2CO3] |
| C��pH=11�İ�ˮ��pH=3������������ϣ�[Cl-]=[NH4+]��[OH-]=[H+] |
| D��ij��Һ��ֻ����SO42-��OH-��NH4+��H+�������ӣ�����ܴ��ڣ�[NH4+]��[SO42-]��[H+]��[OH-] |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | K+ | Na+ | NH4+ | SO42- | NO3- | Cl- | ||
| 4��10-6 | 6��10-6 | 2��10-5 | 4��10-5 | 3��10-5 | 2��10-5 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com