
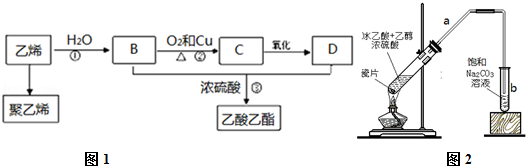
 ��
������ ��ϩ��ˮ�����ӳɷ�Ӧ����B��BΪCH3CH2OH��B������������C��CΪCH3CHO��C����������D��DΪCH3COOH��B��D����������Ӧ����������������ϩ�����Ӿ۷�Ӧ���ɾ���ϩ���Դ˽����⣮
��� �⣺��ϩ��ˮ�����ӳɷ�Ӧ����B��BΪCH3CH2OH��B������������C��CΪCH3CHO��C����������D��DΪCH3COOH��B��D����������Ӧ����������������ϩ�����Ӿ۷�Ӧ���ɾ���ϩ��
��1������ϩ�Ľṹ��ʽΪΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2���Ҵ���ͭ�������¿ɱ�����������ȩ������ʽΪ2CH3CH2OH+O2$��_{��}^{Cu/Ag}$2CH3CHO+2H2O��
������Ҵ���Ũ���������¼��ȷ���������Ӧ����������������Ӧ�ķ���ʽΪCH3COOH+CH3CH2OH$?_{��}^{Ũ����}$CH3COOCH2CH3+H2O��ҲΪȡ����Ӧ��
�ʴ�Ϊ��2CH3CH2OH+O2$��_{��}^{Cu/Ag}$2CH3CHO+2H2O��������Ӧ��CH3COOH+CH3CH2OH$?_{��}^{Ũ����}$CH3COOCH2CH3+H2O�� ȡ����Ӧ/������Ӧ��
��3���Թ������Ƭ�������Ƿ�ֹҺ�屩�У�����a�������ǵ����� �����������Թ�b�ڱ���Na2CO3��Һ������һ�����dz�ȥ���������л��е�������Ҵ�����һ�����ǽ��������������ܽ�ȣ�
�ʴ�Ϊ����ֹҺ�屩�У��������������������������ܽ�ȣ�
���� ���⿼���л����ƶϣ�Ϊ��Ƶ���㣬������ѧ���ķ��������Ŀ��飬�漰ϩ��������ȩ�����ᡢ��֮���ת������ȷ�����ż������ʹ�ϵ���ɽ��ע�ⷴӦ��������Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����۵�ĸߵͣ�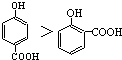 | |
| B�� | Ӳ���ɴ�С�����ʯ��̼���裾����� | |
| C�� | �۵��ɸߵ��ͣ�Na��Mg��Al | |
| D�� | �������ɴ�С��NaF��NaCl��NaBr��NaI |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CaC2 | B�� | H2O | C�� | C2H4 | D�� | H2O2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
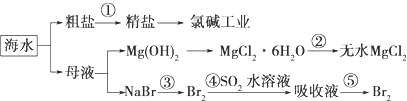
| A�� | �ڢٲ��г�ȥ�����е�SO42-��Ca2+��Mg2+��Fe3+�����ʣ������ҩƷ˳��Ϊ��Na2CO3��Һ��NaOH��Һ��BaCl2��Һ-�����˺������ | |
| B�� | �ӵڢ۲����ڢݲ���Ŀ����Ũ���������� | |
| C�� | �ڢٵ��ڢݲ��У��漰��������ԭ��Ӧ����2�� | |
| D�� | �ڢܲ��У�SO2���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���� | B�� | �������� | C�� | ��֬ | D�� | ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����Na�۷е���ڽ���K | |
| B�� | �ǽ�����N��P�����ǵ��ʻ����ԣ����������� | |
| C�� | ��ͬ�����£�NH3��ˮ�е��ܽ�ȴ���PH3 | |
| D�� | SiC������۵�Ⱦ����ĸ� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com