
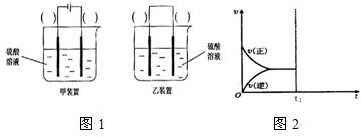
| �� |
| Cu2O |
| ʱ��/min | 20 | 40 | 60 | 80 |
| n��O2��/mol | 0.0010 | 0.0016 | 0.0020 | 0.0020 |
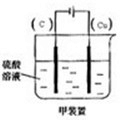 ��
�� ��2H++2e-��H2����
��2H++2e-��H2����| �� |
| Cu2O |
| c2(H2)?c(O2) |
| c2(H2O) |
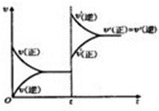 ��
�� ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
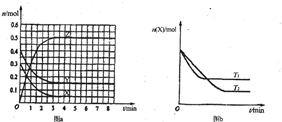 ��2010?�����ģ�⣩T0��ʱ����2L���ܱ������з�����Ӧ��aX��g��+bY��g��?cZ��g���������ʵ����ʵ�����ʱ��仯�Ĺ�ϵ��ͼa��ʾ������������ͬ���¶ȷֱ�ΪT1�桢T2��ʱ������Ӧ��X�����ʵ�����ʱ��仯�Ĺ�ϵ��ͼb��ʾ��������������ȷ���ǣ�������
��2010?�����ģ�⣩T0��ʱ����2L���ܱ������з�����Ӧ��aX��g��+bY��g��?cZ��g���������ʵ����ʵ�����ʱ��仯�Ĺ�ϵ��ͼa��ʾ������������ͬ���¶ȷֱ�ΪT1�桢T2��ʱ������Ӧ��X�����ʵ�����ʱ��仯�Ĺ�ϵ��ͼb��ʾ��������������ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com