
����Ŀ����Դ�������������������ᷢչ������ء�
��1����ҵ������CO��H2�ڴ��������ºϳɼ״���CO(g)+2H2(g) ![]() CH3OH(g) ����֪��Ӧ���й����ʵĻ�ѧ�������������±���ʾ��
CH3OH(g) ����֪��Ӧ���й����ʵĻ�ѧ�������������±���ʾ��
��ѧ�� | H��H | C��O | C��O | H��O | C��H |
E/(kJ/mol) | 436 | 343 | 1076 | 465 | 413 |
�� CO(g)+2H2(g) ![]() CH3OH(g) ��H=__________kJmol-1
CH3OH(g) ��H=__________kJmol-1
��2���Լ״�Ϊȼ�ϵ����͵��,��ɱ�������������Ϊȼ�ϵĴ�ͳȼ�ϵ�ء���ͼ��Ŀǰ�о��϶��һ�����������ȼ�ϵ�ع���ԭ��ʾ��ͼ��

B��Ϊ���______����B���ĵ缫��ӦʽΪ_________________________________��
��3���ο��ϳɷ�ӦCO(g)+2H2(g) ![]() CH3OH(g)��ƽ�ⳣ�����ش��������⣺
CH3OH(g)��ƽ�ⳣ�����ش��������⣺
�¶�/�� | 0 | 50 | 100 | 200 | 300 | 400 |
ƽ�ⳣ�� | 667 | 100 | 13 | 1.9��10-2 | 2.4��10-4 | 1��10-5 |
�ٸ÷�Ӧ����Ӧ�� ___________��������������������������Ӧ��
����T��ʱ��1L�ܱ������У�Ͷ��0.1molCO��0.2molH2���ﵽƽ��ʱ��COת����Ϊ50%����T=__________�档
��4��CH3OHҲ����CO2��H2�ϳɡ������Ϊ1L���ܱ������У�����lmolCO2��3molH2��һ�������·�Ӧ��CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ��H= �� 49.0kJ/mol�����CO2��CH3OH(g)Ũ����ʱ��仯��ͼ��ʾ��
CH3OH(g)+H2O(g) ��H= �� 49.0kJ/mol�����CO2��CH3OH(g)Ũ����ʱ��仯��ͼ��ʾ��
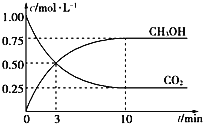
�ٸ÷�Ӧ��ƽ�ⳣ������ʽΪK=_______���ӷ�Ӧ��ʼ��10min��v(H2)=_____mol/(L��min)��
�����������˵���÷�Ӧһ���ﵽƽ��״̬����___________(����ĸ)
A��v(CO2)���� = v(CH3OH)����
B��������ܶȲ�����ʱ��ı�
C��CO2��CH3OH��Ũ��֮�Ȳ�����ʱ��ı�
D�������ƽ����Է�������������ʱ��ı�
��Ϊ�˼ӿ컯ѧ��Ӧ������ʹ��ϵ����������ʵ�������ֻ�ı�����ijһ�������ɲ�ȡ�Ĵ�ʩ��__ (����ĸ)
A�������¶� B����С�������
C���ٳ���CO2���� D��ʹ�ú��ʵĴ���
����ͬ�¶��£�����һ���ݻ�Ϊ1 L���ܱ������г���2mol CH3OH(g)��2molH2O(g)���ﵽƽ��ʱCO2��Ũ��_______(����>������ <������=��)0.25mol/L��
���𰸡�-99 �� CH3OH + 3O2��-6e��= CO2 + 2H2O ���� 50 ![]() 0.225 CD AC >
0.225 CD AC >
��������
��1�����ݡ�H=��Ӧ������ܺͣ�����������ܺ�=(1076��2��436��3��413��343��465)kJ��mol��1=��99kJ��mol��1��
��2������O2���ƶ�����A��Ϊ������B��Ϊ������B�缫��ӦʽΪCH3OH��3O2��=6e��=CO2��2H2O��
��3���ٻ�ѧƽ��ֻ���¶ȵ�Ӱ�죬���ݱ����е����ݣ������¶ȵ����ߣ���ѧƽ�ⳣ�����ͣ�˵�������¶�ƽ�����淴Ӧ�����ƶ���������ӦΪ���ȷ�Ӧ��
�� CO(g)+2H2(g) ![]() CH3OH(g)
CH3OH(g)
��ʼ�� 0.1 0.2 0
�仯�� 0.05 0.1 0.05
ƽ�⣺ 0.05 0.1 0.05 K=![]() =100�����ݱ������ݣ���ʱ�¶�Ϊ50�棻
=100�����ݱ������ݣ���ʱ�¶�Ϊ50�棻
��4���ٸ��ݻ�ѧƽ�ⳣ���ı���ʽ���÷�Ӧ��ƽ�ⳣ��K=![]() �����ݻ�ѧ��Ӧ���ʵ���ѧ����ʽ��v(H2)=0.75��3/(1��10)mol/(L��min)=0.225mol/(L��min)��
�����ݻ�ѧ��Ӧ���ʵ���ѧ����ʽ��v(H2)=0.75��3/(1��10)mol/(L��min)=0.225mol/(L��min)��
��A������CO2������CH3OH����Ӧ����������Ӧ������У�v(CO2)���� = v(CH3OH)����������˵����Ӧ�ﵽƽ�⣬��A����
B����ֶ������壬�������������ֲ��䣬����Ϊ���ݣ�����������䣬���ܶȲ��ٸı䣬����˵����Ӧ�ﵽƽ�⣬��B����
C��CO2��CH3OH��Ũ��֮�Ȳ��ٸı䣬˵����Ӧ�ﵽƽ�⣬��C��ȷ��
D����ֶ������壬�����������䣬������Ӧ������У��������ʵ������٣�M=m/n����������ƽ��Ħ���������ٸı䣬˵����Ӧ�ﵽƽ�⣬��D��ȷ��
���������ʵ�������˵����Ӧ���淴Ӧ�����ƶ���A�������¶ȣ��ӿ췴Ӧ��Ӧ���ʣ�����ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ�����淴Ӧ������У���A��ȷ��
B����С�����������ѹǿ����ѧ��Ӧ���ʼӿ죬ѹǿ����ƽ��������Ӧ�����ƶ�����B����
C���ٳ���CO2����Ӧ���ʼӿ죬��Ϊ����������壬����������ʵ�������C��ȷ��
D��ʹ�ô�����ֻ�ӿ췴Ӧ���ʣ��Ի�ѧƽ���ƶ���Ӱ�죬���������ʵ�������ʣ���D����
�����ݢٵ�ƽ�ⳣ���ı���ʽ���ó����¶��»�ѧƽ�ⳣ��K=16/3��
CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g)
CH3OH(g)+H2O(g)
��ʼ�� 0 0 2 2
�仯�� x 3x x x
ƽ�⣺ x 3x 2-x 2-x K=![]() =
=![]() �����x=0.4��CO2�����ʵ���Ũ��Ϊ0.4>0.25��
�����x=0.4��CO2�����ʵ���Ũ��Ϊ0.4>0.25��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1���ڸ�����һ����̼�ɽ���������ԭΪ������
��֪����C(s)��O2(g)===CO2(g)����H1����393.5 kJ��mol��1
��CO2(g)��C(s)===2CO(g)����H2����172.5 kJ��mol��1
��S(s)��O2(g)===SO2(g)����H3����296.0 kJ��mol��1
��д��CO��SO2��Ӧ���Ȼ�ѧ����ʽ__________________________
��2����2 L�̶�������ܱ������ڣ�800��ʱ��Ӧ2NO(g)��O2(g) ![]() 2NO2(g)��ϵ�У�n(NO)��ʱ��ı仯�����
2NO2(g)��ϵ�У�n(NO)��ʱ��ı仯�����
ʱ��(s) | 0 | 1 | 2 | 3 | 4 | 5 |
n(NO)(mol) | 0.020 | 0.010 | 0.008 | 0.007 | 0.007 | 0.007 |
��д���÷�Ӧ��ƽ�ⳣ������ʽ��K��_______________________����֪��K300��>K350������÷�Ӧ��______��Ӧ������ȡ������ȡ�����
����ͼ��ʾNO2�ı仯��������__________����O2��ʾ��0��2 s�ڸ÷�Ӧ��ƽ������v��__________��

����˵���÷�Ӧ�Ѵﵽƽ��״̬����________��
a��v(NO2)��2v(O2) b��������ѹǿ���ֲ���
c��v��(NO)��2v��(O2) d���������ܶȱ��ֲ���
��Ϊʹ�÷�Ӧ�ķ�Ӧ����������ƽ��������Ӧ�����ƶ�����________��
a����ʱ�����NO2���� b���ʵ������¶�
c������O2��Ũ�� d��ѡ���Ч����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й����л����˵����ȷ���ǣ� ��
A. ����ͱ�������Ҫ�Ļ���ԭ�ϣ��ɴ�ʯ�ͷ����л��
B. ���ۡ������ͺ͵����ʶ�����Ȼ�߷��ӻ�����
C. �����£���ˮ�е��ܽ�ȣ��Ҵ�����������
D.  ����ϩ��һ�������¿ɼӾ۷�Ӧ�������Է���ȡ����Ӧ
����ϩ��һ�������¿ɼӾ۷�Ӧ�������Է���ȡ����Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ԷϾ�Ǧ�����еĺ�Ǧ���ϣ�Pb��PbO��PbO2��PbSO4��̿�ڵȣ���H2SO4Ϊԭ�ϣ��Ʊ��ߴ�PbO��ʵ��Ǧ���������á��乤���������£�

��1��Ǧ��̼��ͬ��Ԫ�أ��ұ�̼��4�����Ӳ㣬��Ǧλ��Ԫ�����ڱ���____����___��
��2�����̢��У���Fe2+���£�Pb��PbO2��Ӧ����PbSO4�����ӷ���ʽ��________________��
��3�����̢��У�Fe2+�����̿ɱ�ʾΪ��
i��2Fe2++ PbO2+4H++SO42��2Fe3++PbSO4+2H2O ii: ����
�� д��ii�Ļ�ѧ����ʽ��_________________________��
�� �����ʵ�鷽����֤ʵ���������̡���ʵ�鷽������������
a.���ữ��FeSO4��Һ�м���__________��Һ����Һ����ɫ�仯���ټ�������PbO2����Һ��Ϊ________ɫ��
b.��a�õ�����Һ�м���___________________,��Һ�ָ�ԭ������ɫ��
��4������II�й��˲�������Ҫ�IJ���������__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ѫ֬��һ�ֳ�������Ѫ�ܼ��������Ƹ�Ѫ֬����ҩI�ĺϳ�·�����£�

��֪��
�ش��������⣺
��1����Ӧ�������Լ��������ֱ���____________��F �Ļ�ѧ����Ϊ____________��
��2���ڵķ�Ӧ������______________��A��B �Ļ�ѧ����ʽΪ_________________��
��3��G �Ľṹ��ʽΪ______________��H �����������ŵ�������____________��
��4��������W ����Է��������Ȼ�����C ��14������������������W �Ŀ��ܽṹ��__�֡�
����FeCl3 ��Һ����ɫ�����ڷ����廯������ܷ���������Ӧ���к˴Ź���������ʾ��5 �ֲ�ͬ��ѧ�������⣬�������Ϊ2:2:2:1:1��д������Ҫ���W �Ľṹ��ʽ____________��
��5������üױ�����ȩΪԭ���Ʊ� �ĺϳ�·�ߣ��������Լ���ѡ���ϳ�·�߳��õı�ʾ��ʽΪ��
�ĺϳ�·�ߣ��������Լ���ѡ���ϳ�·�߳��õı�ʾ��ʽΪ�� ��____________��
��____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ3��ʵ��װ�ã��ֱ�ش��������⡣

��1��װ��1Ϊ����������ʴʵ�顣�����̼���IJ���Ͳ�ڵ����̪��Һ���ɹ۲쵽̼����������Һ��죬�õ缫��ӦʽΪ___________________��
��2��װ��2�����Ϊ�Ȼ�����Һ���ҳ�Ϊ����ͭ��Һ��һ��ʱ����ҳ���Һ��c��Cu2+��_________(���������С�����䡱)������װ�õ�������ʢװ����NH4NO3����֬��Һ���������е�_____���ӣ��NH4+����NO3���������Ȼ�����ҺǨ�ơ�
��3��װ��3�м��ձ�ʢ��100mL 0.2mol/L��NaCl��Һ�����ձ�ʢ��100mL 0.5mol/L��CuSO4��Һ����Ӧһ��ʱ��۲쵽���ձ���ʯī�缫�����������ɡ�
�ٵ�Դ��M��Ϊ___�������ձ������缫�ĵ缫��ӦʽΪ_______________��
�����ձ��е�ⷴӦ�����ӷ���ʽΪ__________________________________��
��ֹͣ��⣬ȡ��Cu�缫��ϴ�ӡ�����������缫����0.32g�����ձ��в����������״�������Ϊ________mL���������ձ���Һ��pHΪ______��������ǰ����Һ��������䣩��
����Ҫ���ҳ���Ƴɵ�⾫��ͭ��װ�ã����ҳص�ʯīӦ�ij�_____�����ͭ����ͭ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ֻ��һ���Լ��Ϳ��Լ���������Һ����������Һ��������Һ�������Լ��ǣ� ��
A. NaOH��ҺB. Cu(OH)2����ҺC. ��ˮD. Na2CO3��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����A��B��C��D��E����Ԫ�أ��������Ϣ�����
Ԫ�� | �����Ϣ |
A | Aԭ�ӵ�1s�����ֻ��1������ |
B | B�ǵ縺������Ԫ�� |
C | C��̬ԭ�ӵ�2p�������3��δ�ɶԵ��� |
D | D������Ԫ������Eͬ���ڣ��������ܲ�����2���˶�״̬��ͬ�ĵ��� |
E | E���γɺ�ɫ(��ש��ɫ)��E2O�ͺ�ɫ��EO���������� |
��ش��������⣺
��1��д��EԪ��ԭ�ӻ�̬ʱ�ĵ����Ų�ʽ_______________��
��2��CԪ�صĵ�һ�����ܱ���Ԫ�صĵ�һ������_______________ (���С��) ��
��3��CA3������Cԭ�ӵ��ӻ�������_______________��
��4��A��C��E����Ԫ�ؿ��γ�[E(CA3)4]2+�����д��ڵĻ�ѧ��������____(�����)��
����λ�� �ڽ����� �ۼ��Թ��ۼ� �ܷǼ��Թ��ۼ� �����Ӽ� �����
��[E(CA3)4]2+���жԳƵĿռ乹�ͣ��ҵ�[E(CA3)4]2+�е�����CA3������Clȡ��ʱ���ܵõ����ֲ�ͬ�ṹ�IJ����[E(CA3)4]2+�Ŀռ乹��Ϊ____________ (�����)��
a.ƽ�������� b.�������� c.������ d.V��
��5��B��D���γ����ӻ�����侧���ṹ��ͼ��ʾ������D���ӵ���λ��Ϊ_______________�����þ�����ܶ�Ϊa g��cm3����þ����������_____________cm3(д������ʽ����)��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����Ź�ҵ�ķ�չ�������Ѿ���Ϊȫ���ԵĻ������⡣
��1������ˮ��pH___________�����dz���Ϊ���ꡣ
��2���û�ѧ����ʽ����ʾ�����γɵ���Ҫ;��֮һ��_____________________��_______________��
��3�������²��ij��������Ʒ��pH=5�������Ʒ��c(H+)=_____________������Ʒ����һ��ʱ�������������ǿ����ԭ�������________________________________________________��
��4��д��һ�����ٶ��������ŷŵĴ�ʩ_________________________________________��
��5������ij��ɫ��Һ���Ƿ����SO42-�ķ�����_____________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com