
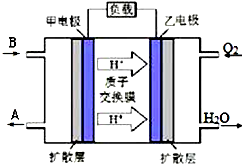
 ��֪A��B��C��D����ѧ��ѧ�������ʣ�������һ����������A+B��C+D��ת����ϵ��
��֪A��B��C��D����ѧ��ѧ�������ʣ�������һ����������A+B��C+D��ת����ϵ��
| ||
| �� |
| ||
| �� |
| ŨH2SO4 |
| �� |
| ŨH2SO4 |
| �� |


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
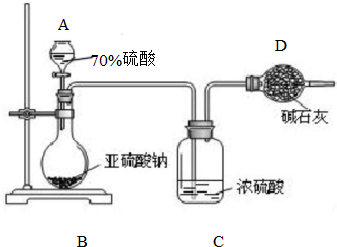 Ϊ�ⶨij����������Ʒ�Ĵ��ȣ���ͬѧ��ȡ10.0g���壬��������ʵ�飺
Ϊ�ⶨij����������Ʒ�Ĵ��ȣ���ͬѧ��ȡ10.0g���壬��������ʵ�飺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
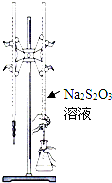 H2S2O3��һ�����ᣬʵ��������0.01mol?L-1��Na2S2O3��Һ�ζ�I2��Һ�������ķ�ӦΪI2+2Na2S2O3=2NaI+Na2S4O6������˵���������ǣ�������
H2S2O3��һ�����ᣬʵ��������0.01mol?L-1��Na2S2O3��Һ�ζ�I2��Һ�������ķ�ӦΪI2+2Na2S2O3=2NaI+Na2S4O6������˵���������ǣ�������| A���õζ����ü�����ָʾ�� |
| B��Na2S2O3�Ǹ÷�Ӧ�Ļ�ԭ�� |
| C���õζ���ѡ����ͼ��ʾװ�� |
| D���÷�Ӧ��ÿ����2mol Na2S2O3������ת����Ϊ4mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
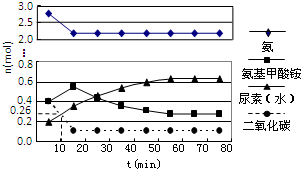 ���أ�H2NCONH2����һ�ַdz���Ҫ�ĸߵ����ʣ���ҵ�Ϻϳ����صķ�Ӧ��Ϊ����������
���أ�H2NCONH2����һ�ַdz���Ҫ�ĸߵ����ʣ���ҵ�Ϻϳ����صķ�Ӧ��Ϊ�����������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������CH4��Cl2�ڹ����·�Ӧ���ɴ�����CH3Cl |
| B��������Ũ�����Ũ���Ṳ����ȡ������ |
| C�������ȵ�ͭ˿Ѹ�ٲ�����ˮ�Ҵ��пɽ��Ҵ�����Ϊ��ȩ |
| D������Ũ������������Һ�ͼ�������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ��� |
| ||
| ||
| ||

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
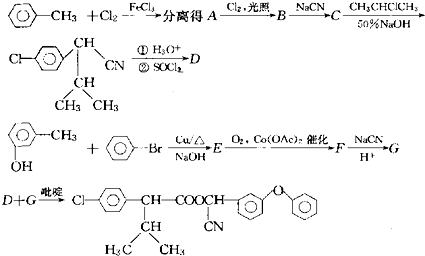
| ��H3O+ |
| ��SOCl2 |
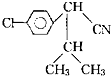
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��CCl4����CH4�Ƶã�����ȡ��ˮ�еĵ� |
| B��ʯ�ͺ���Ȼ������Ҫ�ɷֶ���̼�⻯���� |
| C��������ʹ����KMnO4��Һ��ɫ����˱����ܷ���������Ӧ |
| D�����ۺ���ά�صĻ�ѧʽ��Ϊ��C6H10O5��n��������ͬ���칹�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com