
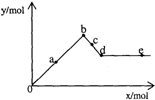
 ��֪Ba��AlO2��2������ˮ����ͼ��ʾ������A12��SO4��3��Һ����μ���Ba��OH��2��Һʱ�����ɳ��������ʵ���y�����Ba��OH��2�����ʵ���x�Ĺ�ϵ�������й�������ȷ���ǣ�������
��֪Ba��AlO2��2������ˮ����ͼ��ʾ������A12��SO4��3��Һ����μ���Ba��OH��2��Һʱ�����ɳ��������ʵ���y�����Ba��OH��2�����ʵ���x�Ĺ�ϵ�������й�������ȷ���ǣ�������| A��a-bʱ���������ʵ�����A1��OH��3��BaSO4�� |
| B��c-dʱ��Һ�����ӵ����ʵ�����AlO2-��Ba2+�� |
| C��a-dʱ���������ʵ�����BaSO4����С��A1��OH��3 |
| D��d-eʱ��Һ�����ӵ����ʵ�����Ba2+�����ܵ���OH- |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��0.4 mol |
| B��0.6 mol |
| C��0.8 mol |
| D��1.2 mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
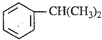 ������һ����Ҫ���л�����ԭ�ϣ������������������գ�
������һ����Ҫ���л�����ԭ�ϣ������������������գ�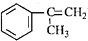 ��������������ABS��֬��һ�ֵ��壬��ҵ���������������õ���д�����������ȡ�õ������һ�ַ���
��������������ABS��֬��һ�ֵ��壬��ҵ���������������õ���д�����������ȡ�õ������һ�ַ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | ������ƽ | С�ձ� | ����ǯ | ������ | ҩ�� | ��Ͳ |
| ���� |  |  |  |  |  | |
| ��� | a | b | c | d | e | f |
| ʵ�� ���� | ��ʼ�¶�t1/�� | ��ֹ�¶� t2/�� | �¶Ȳ�ƽ��ֵ ��t2-t1��/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 30.1 | |
| 2 | 27.0 | 27.4 | 27.2 | 33.3 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.8 | |
| 4 | 26.4 | 26.2 | 26.3 | 30.4 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������������ ������̼ CO2 |
| B���� ���� H2S |
| C������������ �������� Ca��OH��2 |
| D���� ���� NaOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����װ��ҩƷ���ټ��װ�õ������� |
| B������˫������Թܣ��ٽ����ܲ���ˮ�м��װ�õ������� |
| C��������ԭ����ͭ��ʵ�飬��ͨһ��ʱ���������������������ȣ��ټ��� |
| D��ϡ��Ũ����ʱ�������ձ��ﵹ��Ũ���ᣬ��С�ĵ���ˮ�����Ͻ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �ŵ� |
| ��� |
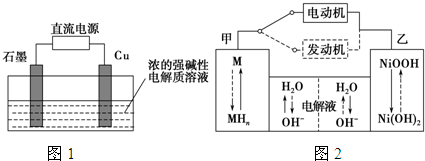
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ᳫ˫��ʹ��ֽ�� |
| B���ƹ�һ������� |
| C���ᳫʹ��̫������ˮ�� |
| D���ƹ㹫�����г� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com