
��100 mL FeI2��Һ����ͨ��Cl2������������I2��Fe3����IO3-������Fe3����I2�����ʵ�����n(Cl2)�ı仯��ͼ��ʾ����ش��������⣺
��1����ͼ��֪��I����Fe2����I2�������ӵĻ�ԭ����ǿ������˳��Ϊ________��________��________��
��2����n(Cl2)��0.12 molʱ����Һ�е�������ҪΪ________________________________��
�ӿ�ʼͨ��Cl2��n(Cl2)��0.12 molʱ���ܷ�Ӧ�Ļ�ѧ����ʽΪ______________________��
��3������Һ��n(Cl��)��n(IO3-)��8��1ʱ��ͨ���Cl2�ڱ�״���µ����Ϊ________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ԭ����ͭ���õĺ�ɫ���������ͭ��������ͭ�Ļ�����֪Cu2O��������Һ�пɷ�������������ԭ��Ӧ������Cu2+��Cu��
��1������8.4������ͭ��������ȫ��ԭ�õ���ɫ����6.96�ˣ����к�����ͭ��������ͭ�����ʵ���֮���� ��
��2������6.96�������������������Ũ�����ַ�Ӧ��
�����ɱ�״����1.568�������壨������NO2���ܽ⣬Ҳ������NO2��N2O4��ת�������������ijɷ��� �������ʵ���֮���� ��
�ڰѵõ�����ҺС������Ũ�����������ľ�����ˣ��þ���20.328g����������ԭ��Һ�е�Cu2+��20%������ĸҺ�У����þ���Ļ�ѧʽΪ ��
��3��Cu��Cu2O��CuO��ɵĻ�������100mL 0.6mol/L HNO3��Һǡ��ʹ�������ȫ�ܽ⣬ͬʱ�ռ���224mL NO����(��״��)����ԭ�������Cu�����ʵ���ΪX��������Cu2O��CuO�����ʵ�����X��ȡֵ��Χ(д���������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ǿ���������Ǹ��ֽⷴӦ��һ����Ҫ���ɡ�����ġ�ǿ�ᡱ�������ᡱָ���ǿ�����ܳ������Ե�һЩ���������ʣ�����ࡢ�������������ʽ�εȲ���ķ�ӦҲ�ɸ���������ǿ�����������������ж�����
(1)HA��H2B���������ᣬ�����¹�ϵ��H2B(����)��2A��=B2����2HA����A����HB����B2�����������У����������(H��)����________��
(2)����ǿ���������ʵı����й��⣬�����ܼ��йأ���CH3COOH��HF��Һ������NH3Ӱ��ɷ�����ȫ���롣��Һ����CH3COONa��HCl�D��NaCl��CH3COOH��һ��Ӧ�ܷ���________(��ܡ���)��������____________________��
(3)ijͬѧʵ�鷢�֣���H2S����ͨ��CuSO4��Һ�У����ɺ�ɫ������Ū�������CuS��д���˻�ѧ����ʽ��H2S��CuSO4=CuS����H2SO4��������������������ⲻ�������Ƶ�ǿ��������ǿ��������Ĺ���ì���ˡ������������__________________________________________��
(4)������ԭ��Ӧ��Ҳ�����ƹ��ɣ���ǿ�����������������������ʡ�����ǿ��ԭ������������ԭ�����ʡ����ݴ��ж����з�Ӧ�ܹ���������________(����ĸ���)��
A��FeCl2��Cl2 FeCl3
FeCl3
B��Fe��I2 FeI3
FeI3
C��Fe��CuSO4 FeSO4��Cu
FeSO4��Cu
D��FeCl3��Cu CuCl2��FeCl2
CuCl2��FeCl2
E��FeBr3��Cl2 FeCl2��Br2
FeCl2��Br2
F��FeI2��Br2 FeBr3��I2
FeBr3��I2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ĺ���������ҽҩ�������ȷ���������Ҫ����;��
��1���ؾ�ʯ��BaSO4������θ������Ӱ����
��֪�������£�Ksp��BaSO4����1.1��10��10����BaSO4����Һ�м������ᣬ����Һ��pH��2ʱ����Һ��c��Ba2������ __��
��2����������茶���[��NH4��2Fe��SO4��2��6H2O]������������
�ټ��龧���к���NH4+�ķ���Ϊ ��
�ڵ����ʵ���Ũ�ȵ�����ϡ��Һ��
a����NH4��2Fe��SO4��2 b��NH4HSO4
c����NH4�� 2SO4 d����NH4��2SO3��
����c��NH4+���ɴ�С��˳��Ϊ __����ѡ����ĸ����
��3����������أ�K2S2O8������ǿ��������Na2S2O3������ԭ����
��K2S2O8��Һ������MnSO4��Һ��ϣ��ڴ��������£����Թ۲쵽��Һ��Ϊ��ɫ���÷�Ӧ�����ӷ���ʽΪ __��
���ò����缫�����H2SO4��K2SO4�Ļ����Һ�����Ʊ�K2S2O8���������ĵ缫��ӦʽΪ __��������������������Һ��pH�� __���������С�����䡱����
�۲�Ʒ��K2S2O8�ĺ������õ������ⶨ����������Ϊ��ȡ0.3000 g��Ʒ�ڵ���ƿ�У���50 mLˮ�ܽ⣻����4.000 g KI���壨�Թ���������ʹ���ַ�Ӧ����������������Һ�ữ���� __Ϊָʾ������0.1000 mol��L��1 Na2S2O3��Һ�ζ����յ㣨��֪��I2��2S2O32-=2I����S4O62-�����ظ�2�Σ����ƽ�����ı�Һ21.00 mL���ò�Ʒ��K2S2O8����������Ϊ�����ʲ��μӷ�Ӧ�� __����ʽ�����㣩��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����һ�ֻ��ý����������ܶ�С���۵�ߡ�������ǿ����еǿ�ȸߵ����ܡ���ҵ�ϳ�������ֽ����ѿ�ʯ���Ʊ��������ѣ�����ұ���ѣ���Ҫ�����������Ӧ��
��FeTiO3��2H2SO4=TiOSO4��FeSO4��2H2O
��TiOSO4��2H2O=H2TiO3����H2SO4
��H2TiO3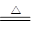 TiO2��H2O
TiO2��H2O
��TiO2��2C��2Cl2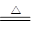 TiCl4����2CO��
TiCl4����2CO��
��TiCl4��2Mg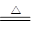 2MgCl2��Ti
2MgCl2��Ti
(1)������������Ӧ�����������������________��
A����Ӧ���Ƿ�������ԭ��Ӧ
B����Ӧ��������������
C����Ӧ���е�TiO2��������
D����Ӧ�ݱ����˽���þ�Ƚ����ѵĻ�ԭ��ǿ
(2)�Ѿ��к�ǿ����ʴ�ԣ����¶���ԭ��ķ�����ȷ����________��
A��������𡢲�һ���IJ����ý���
B�������ѵı������γ����ܵ�����Ĥ
C��������������ȸ�ʴ������Ӧ
D�����кܸߵ�ǿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ǵؿ��к�����Ϊ�ḻ�ķǽ���Ԫ�أ���Ҫ��������ˮ����������Ca3(PO4)2����ʽ���ڡ����ĵ��ʺͻ������ڹ�ũҵ������������Ҫ��Ӧ�á�
(1)����(P4)����Ca3(PO4)2����̿��SiO2��һ�������·�Ӧ��á�����Ȼ�ѧ����ʽ���£�
2Ca3(PO4)2(s)��10C(s)=6CaO(s)��P4(s)��10CO(g)�� ��H1����3359.26 kJ��mol��1
CaO(s)��SiO2(s)=CaSiO3(s) ��H1����89.61 kJ��mol��1
2Ca3(PO4)2(s)��6SiO2(s)��10C(s)=6CaSiO3(s)��P4(s)��10CO(g)�� ��H3
��H3��________kJ��mol��1��
(2)�����ж������CuSO4��Һ�ⶾ���ⶾԭ���������л�ѧ����ʽ��ʾ��
11P4��60CuSO4��96H2O=20Cu3P��24H3PO4��60H2SO4
60 mol CuSO4�������������ʵ�����________��
(3)����Ҫ������NaH2PO4��Na2HPO4��Na3PO4��ͨ��H3PO4��NaOH��Һ��Ӧ��ã��������ֵķֲ�����(ƽ��ʱij���ֵ�Ũ��ռ������Ũ��֮�͵ķ���)��pH�Ĺ�ϵ����ͼ��ʾ��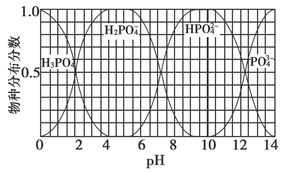
��Ϊ��þ����ܴ���NaH2PO4��pHӦ������________��pH��8ʱ����Һ����Ҫ��������Ũ�ȴ�С��ϵΪ________��
��Na2HPO4��Һ�Լ��ԣ�������Һ�м���������CaCl2��Һ����Һ�������ԣ���ԭ����________(�����ӷ���ʽ��ʾ)��
��4���Ļ������������ף� ���뼾���Ĵ���
���뼾���Ĵ��� �������ʵ���֮��2:1��Ӧʱ���ɻ��һ��������ȼ���м���X�����ͷų�һ���������塣�����Ĵ���X�ĺ˴Ź�����������ͼ��ʾ��
�������ʵ���֮��2:1��Ӧʱ���ɻ��һ��������ȼ���м���X�����ͷų�һ���������塣�����Ĵ���X�ĺ˴Ź�����������ͼ��ʾ��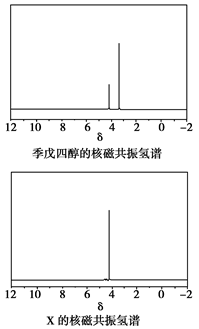
������������______________________(�ѧʽ)��
��X�Ľṹ��ʽΪ__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Cu��һ��Ũ�ȵ�HNO3��ӦΪ:3Cu+2NO3��+xH+ 3Cu2++2R+yH2O��
3Cu2++2R+yH2O��
(1)��Ӧ�е�x=����������������
(2)��Ӧ����R�Ļ�ѧʽΪ����������������
(3)�μӷ�Ӧ��Cu������HNO3�����ʵ���֮��Ϊ������������
(4)1.5 mol Cu��ȫ��Ӧʱת�Ƶĵ�����Ϊ��������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���÷Ͼɶ�п��Ƥ���Ʊ�����Fe3O4�������Ӽ�������ZnO���Ʊ�����ͼ���£�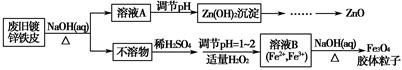
��֪��Zn���仯�����������Al���仯������������ơ���ش��������⣺
��1����NaOH��Һ�����Ͼɶ�п��Ƥ��������________��
| A��ȥ������ | B���ܽ��п�� | C��ȥ������ | D���ۻ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1��������FeS2������������Ŀ���ԭ�ϣ���Ӧ����ΪFeS2��SO2��SO3��H2SO4����д��
SO2�Ʊ�SO3��Ӧ�Ļ�ѧ����ʽ������˫���ű������ת�Ƶķ������Ŀ ��
��2�����������������FeS2��������Ӧʱ������S��NԪ�صĻ��ϼ۲��ᷢ���仯���� ��
a��ϡ���� b��ϡ���� c��Ũ���� d��Ũ����
��3���ӿ���ѧ���ϲ�ã���Ȼ����ڷ�Ӧ14CuSO4+5FeS2+12H2O=7Cu2S+5FeSO4+12H2SO4��
�÷�Ӧ��������Ϊ ����Ӧ��ת�Ƶĵ�����Ϊ NA��
��4������2���з�Ӧ�IJ�������ˮ����ˣ�Cu2S������ˮ��ϡ�ᣩ���ٽ���Һ�����������ữ�ĸ��������Һ�з�����Һ��ɫ����֪��Ӧ����Ԫ�س�+2�ۣ���д����Ӧ�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com