
����Ŀ��һ�������£��������ʵ�����A��B�������2 L���ܱ������У��������·�Ӧ��3A(g)��B(g) ![]() mC(g)��2D(g)������5 min��Ӧ�ﵽƽ�⡣��ʱ��ã�D��Ũ��Ϊ0.5 mol/L��c(A)��c(B)��1��3��C�ķ�Ӧ������0.1 mol��L��1��min��1����
mC(g)��2D(g)������5 min��Ӧ�ﵽƽ�⡣��ʱ��ã�D��Ũ��Ϊ0.5 mol/L��c(A)��c(B)��1��3��C�ķ�Ӧ������0.1 mol��L��1��min��1����
��m��ֵΪ���٣�_____________
��A��5 minĩ��Ũ�ȡ�__________
��B��ƽ��ת���ʡ�___________
�ܸ÷�Ӧ��ƽ�ⳣ����___________
���𰸡�m=2 0.25 mol��L��1 25% ![]()
��������
��D��Ũ��Ϊ0.5mol��L��1����D�ķ�Ӧ����Ϊ![]() =0.1 mol��L��1��min��1�����ݻ�ѧ��Ӧ����֮�ȵ��ڼ�����֮��0.1 mol��L��1��min��1��0.1 mol��L��1��min��1=m:2����m=2��
=0.1 mol��L��1��min��1�����ݻ�ѧ��Ӧ����֮�ȵ��ڼ�����֮��0.1 mol��L��1��min��1��0.1 mol��L��1��min��1=m:2����m=2��
���跴Ӧ��ʼǰ����������A��B���ʵ���Ũ��Ϊa����
3A(g)��B(g) ![]() 2C(g)��2D(g)
2C(g)��2D(g)
��ʼ��Ũ�ȣ��� a a 0 0
���ģ�Ũ�ȣ��� 0.75 0.25 0.5 0.5
ƽ�⣨Ũ�ȣ���(a��0.75) (a��0.25) 0.5 0.5
��Ϊc(A)�Uc(B)=1:3������a-0.75��:��a-0.25��=1:3�����a=1mol/L����A��B���ʵ���Ũ�ȶ�Ϊ1mol/L��
A��5 minĩ��Ũ��Ϊ��a��0.75��mol/L =1mol/L-0.75mol/L=0.25mol/L��
��B��ƽ��ת����Ϊ![]() ��
��
�ܸ÷�Ӧ��ƽ�ⳣ��K=![]() =
=![]() ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼ��ֱ��ʾ�йط�Ӧ�ķ�Ӧ�����������仯�Ĺ�ϵ���ݴ��ж�����˵����ȷ���� �� ��
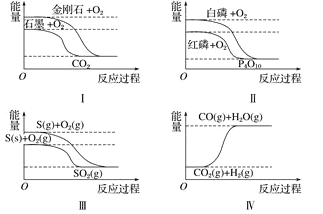
A.���ʯ��ʯī�ȶ�
B.����ת��Ϊ���������ȷ�Ӧ
C.S(g)+O2(g) === SO2(g)����H1 ��S(s)+O2(g) === SO2(g)����H2������H1>��H2
D.CO(g)+H2O(g) === CO2(g)+H2(g)����H>0
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ӧ4NH3��������5O2������![]() 4NO��������6H2O��������10L�ܱ������н��У�����Ӻ�ˮ���������ʵ���������0.45mol����˷�Ӧ��ƽ�������� (X)(��Ӧ����������ʻ�������������)�ɱ�ʾΪ( )
4NO��������6H2O��������10L�ܱ������н��У�����Ӻ�ˮ���������ʵ���������0.45mol����˷�Ӧ��ƽ�������� (X)(��Ӧ����������ʻ�������������)�ɱ�ʾΪ( )
A. ��(NH3)=0.0100mol��L-1��s-1B. ��(O2)=0.0010 mol��L-1��s-1
C. ��(NO)=0.0010 mol��L-1��s-1D. ��(H2O)=0.045 mol��L-1��s-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��W��X��Y��Z���ֶ�����Ԫ�أ���Ԫ�����ڱ��е�λ����ͼ��ʾ������WԪ�ص�ԭ������ΪZԪ��ԭ��������������������˵����ȷ����
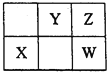
A. Xλ��Ԫ�����ڱ��еĵ�3���ڵڢ�A��
B. X��Y��Z����Ԫ�ض�Ӧԭ�ӵİ뾶���μ�С
C. XZ2��YZ2�Ľṹ�ͻ�ѧ��������
D. ����Y�ĺ���������ǿ��W�ĺ��������ԣ���֤���ǽ�����Wǿ��Y
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���100mL0.01molL-1HA��Һ����μ���0.02molL -1MOH��Һ��ͼ����ʾ���߱�ʾ�����Һ��pH�仯�������仯���Բ���)������˵���в���ȷ����( )

A.MOHΪһԪ����
B.MAϡ��ҺpH<7
C.N��ˮ�ĵ���̶ȴ���K��ˮ�ĵ���̶�
D.K���Ӧ����Һ��pH=10����c(MOH)+c(OH)-c(H+)=0.01molL-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����2L�ĺ����ܱ������У�ͨ��4mol A��5mol B��������Ӧ��4A(g)��5B(g)��3C(g)��3D(s)��5min��ѹǿ��Ϊԭ����80%����÷�Ӧ��0��5min�ڵ�ƽ����Ӧ���ʿɱ�ʾΪ
A.v(A)��0.24 mol��L��1��min��1B.v(B)��0.15 mol��L��1��min��1
C.v(C)��0.18 mol��L��1��min��1D.v(D)��0.36 mol��L��1��min��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͬ��ͬѹ�£���3֧��ͬ������Թ��зֱ���е�������������壺��NO2����HC1����NH3���ֽ�3֧�Թܾ�������ˮ���У�����ܽ��������Һ�����ʵ���Ũ�ȴ�С��ϵ��ȷ����
A.��>��>��B.��=��=��C.��=��>��D.��>��>��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��DΪʯī�缫��E��F�ֱ�Ϊ�������������ֻ��ý����е�һ�֣���E����NaOH��Һ��Ӧ����ͼʾ��ͨ��·����Ӧһ��ʱ�䡣
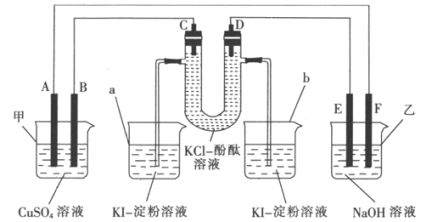
(1)�ж�װ�õ����ƣ��׳�Ϊ_________(��������������ԭ���������ͬ)���ҳ�Ϊ_________��
(2)B��Ϊ_________ (��������������������������������������������ͬ)���缫��ӦʽΪ_________��F��Ϊ_________���缫��ӦʽΪ__________________��
(3)U�ι�����Һ�ȱ�����_________(����C������D��)����U�ι��з�����Ӧ�Ļ�ѧ����ʽΪ________________________________________��
(4)���ձ�����38.1 g I2(KI����)����ʱ���׳�����Һ�����������________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����0.6molA��0.5molB����0.4L�ܱ������з���2A��g��+B��g��![]() mD��g��+E��g��������5min��ﵽ��ѧƽ�⣬��ʱ���DΪ0.2mol����֪5min����E��ʾ��ƽ����Ӧ����Ϊ0.1mol��L-1��min-1�����㣺
mD��g��+E��g��������5min��ﵽ��ѧƽ�⣬��ʱ���DΪ0.2mol����֪5min����E��ʾ��ƽ����Ӧ����Ϊ0.1mol��L-1��min-1�����㣺
��1��m��ֵ___________��
��2��ƽ��ʱB��ת����____________��
��3����ʼ��ƽ��ʱ�ܱ������е�ѹǿ֮��_____________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com