
����Ŀ��Ϊ��ǿ����ȫ������ij�Ϳ�������һ̨���������ͺ����IJ����ǣ��乤��ԭ����ͼ��ʾ (��ǿ������Һ���������Һ)������˵���в���ȷ����
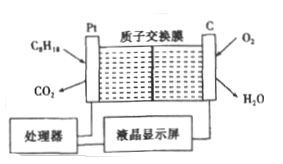
A. ʯ���缫���������缫���������ʱ���ԭ
B. ���缫�ĵ缫��ӦʽΪ: C8H18+16H2O-50e-=8CO2+50H+
C. H+�����ӽ���Ĥ������Ҳ�Ǩ��
D. �����ÿ����5.6 L O2��·��ͨ��1mol ���ӣ�����������γɱպϻ�·
���𰸡�D
��������A����װ����������ȼ�ϵ�أ��������ڵ缫��������������ԭ��Ӧ��A��ȷ��B�����缫������������������ʧȥ���ӱ��������缫��ӦʽΪ��C8H18+16H2O-50e-=8CO2+50H+��B��ȷ��C��ԭ���������������������H+��������Ҳ�Ǩ�ƣ�C��ȷ��D�������ÿ����5.6 LO2����·��ͨ���ĵ��ӵ����ʵ���ӦΪ5.6��22.4��4mol=1mol�������Ӳ���ͨ���������Һ��ֻ���ڵ�����ͨ����D������ȷ��ΪD��


 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������л����У��������������������(����)
A��±���� B������
C���ڶ��ױ� D������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ϩ����Ҫ�Ļ���ԭ�ϡ����ұ�(C6H5��CH2CH3)Ϊԭ�ϣ����ô�����ķ�����ȡ����ϩ(C6H5��CH=CH2)����Ӧ����ʽΪ��C6H5��CH2CH3(g)![]() C6H5��CH=CH2(g)+H2(g) ��H=+117.6 kJ��mo1-1
C6H5��CH=CH2(g)+H2(g) ��H=+117.6 kJ��mo1-1
�ش�����������
��1����֪��H2(g)+1/2O2(g)=H2O(l) ��H=-285.8 kJ��mo1-1
C6H5��CH2CH3(g)+21/2O2(g)=8CO2(g)+5H2O(l) ��H=-4607.1 kJ��mo1-1
��C6H5��CH=CH2(g)+10O2(g)= 8CO2(g)+4H2O(l) ��H=_____________��
��2����ҵ�ϣ��ں�ѹ�豸�н���������Ӧ��ȡ����ϩ�������ұ�������ͨ�����ˮ���������û�ѧƽ�����۽���ͨ�����ˮ������ԭ��_________________________________________��
��3����֪T���£���amol�ұ�����ͨ�뵽���ΪVL���ܱ������н���������Ӧ����Ӧʱ���������ڵ���ѹǿ�������±���
ʱ��t/min | 0 | 10 | 20 | 30 | 40 |
��ѹǿp/1000kPa | 1.0 | 1.3 | 1.45 | 1.5 | 1.5 |
���ɱ������ݼ���0~10 min��v(C6H5��CH2CH3)=________________�����ú�a��V ��ʽ�ӱ�ʾ��
���÷�Ӧƽ��ʱ�ұ���ת����Ϊ_________________________��
��4������ϩ���廯�ⷢ���ļӳɷ�Ӧ���������֣��䷴Ӧ����ʽ������
i.C6H5��CH=CH2(g)+HBr(g)![]() C6H5��CH2CH2Br (g)
C6H5��CH2CH2Br (g)
ii.C6H5��CH=CH2(g)+HBr(g)![]() C6H5��CHBrCH3(g)
C6H5��CHBrCH3(g)
600��ʱ����3L �����ܱ������г���1.2 mol C6H5��CH=CH2(g)��1.2 mol HBr(g)������Ӧ���ﵽƽ��ʱC6H5��CH2CH2Br (g)��C6H5��CHBrCH3(g)�����ʵ���(n)��ʱ��(t)�仯��������ͼ��ʾ��
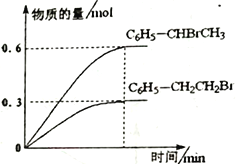
��600��ʱ����Ӧii �Ļ�ѧƽ�ⳣ��K ii=__________________��
����Ӧƽ��������������������䣬����������ٳ���1mol C6H5��CH2CH2Br (g)����Ӧii ��_________(��������������)�ƶ���
���ں��º��ݵ��ܱ������У�����ϩ���廯�ⷢ��i��ii�����ӳɷ�Ӧ���жϷ�Ӧ�Ѵﵽƽ��״̬����______��
B.C6H5��CH2CH2Br (g)������������C6H5��CHBrCH3 (g)�ֽ��������
C.��Ӧ����ѹǿ������ʱ��仯���仯
D.��������ƽ����Է����������ֲ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ƽ�����̣�����������ȵ�A��B���ձ�������ƽ�⣬Ȼ��ֱ���������ȵ��������ᣬ�̶���AB���ձ��з��������������ʣ������ƽ�Ա���ƽ����ǣ���
A. 0.5molNa��0.5molMg B. 0.1molZn��0.1molAl
C. 8.4gMgCO3��8.4gNaHCO3 D. 10gCaCO3��10gMgCO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1�����б仯�У��ٵ����� ���ռ��ۻ� ���Ȼ�������ˮ ���Ȼ�������ˮ �ݹ�����������ˮ ���Ȼ�����ȷֽ� ����������ˮ
���У�������ţ�δ������ѧ���ƻ�����_______�����������Ӽ��ƻ�����______�����������ۼ��ƻ�����_______���ȷ������Ӽ��ƻ����ַ������ۼ��ƻ�����___________��
(2)�������ʵ���A��B�����2L���ܱ�������,�������·�Ӧ��3A(g)+B(g) ![]() xC(g)+2D(g)����5min���D��Ũ��Ϊ0.5mol/L��c(A):c(B)=3:5��C��ƽ����Ӧ������0.1mol/(Lmin)
xC(g)+2D(g)����5min���D��Ũ��Ϊ0.5mol/L��c(A):c(B)=3:5��C��ƽ����Ӧ������0.1mol/(Lmin)
�ٴ�ʱ��A��Ũ��Ϊ__________________��
�ڷ�Ӧ��ʼǰ����������B�����ʵ���Ϊ___________��
��B�Ļ�ѧ��Ӧ����Ϊ_________________________��
��xֵΪ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ԫ�����ڱ��У�����22�ַǽ���Ԫ���⣬����Ķ��ǽ����������Ԫ�����ڱ��ش��������⣺
I��(1)��̬��ԭ�Ӻ����_______���˶�״̬����ͬ�ĵ��ӣ���ԭ�Ӻ�������Ų��е�����������͵Ĺ����_______�Σ���n��ʾ�ܲ㣬��Ԫ�����������Χ�����Ų�ʽΪ______________��
(2)��Ԫ�����ڱ��У�ijЩ����Ԫ�����·�������Ԫ�ص�������Щ���ƣ�����Ϊ���Խ��߹������±���

���ݡ��Խ��߹���д��Be(OH)2��NaOH��Ӧ�����ӷ���ʽ______________������(H3BO3)��һ�־���Ƭ��ṹ�İ�ɫ���壬���ڵ�H3BO3���Ӽ�ͨ�������������ͼ������1mol H3BO3�ľ�������__________mol�����H3BO3��Bԭ�ӵ��ӻ�����Ϊ_____________��

(3)�Եڶ�����Ϊ������Be��N�⣬����Ԫ�صĵ�һ�����ܴ������������ԭ����____________________________________________________��
II�������������仯�����ڹ�ũҵ���й�����Ӧ��ǰ����
(4)����һ��������[Fe(CN)6]4-��Fe2+����λ��Ϊ6�����������в�����______������ţ���
A�����ۼ� B���Ǽ��Լ� C����λ�� D���ļ� E���м�
(5)AlCl3���۵��NaCl�۵�͵�ԭ����____________________________________��
(6)һ��Al-Fe�Ͻ�����徧����ͼ��ʾ����������ܶ�Ϊ�� gcm-3����˺Ͻ������������Feԭ��֮��ľ���Ϊ__________cm���ú����Ĵ���ʽ��ʾ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������MnO2��Ũ�����������ķ�ӦΪMnO2��4HCl(Ũ)![]() MnCl2��2H2O��Cl2������Ӧ�У������17.4 g��MnO2����ԭ����ô��
MnCl2��2H2O��Cl2������Ӧ�У������17.4 g��MnO2����ԭ����ô��
(1)���������Ȼ��������Ϊ________________________________________________��
(2)ת�Ƶ��ӵ����ʵ���Ϊ________________________________________________��
(3)��֯��ҵ�г���������Ư����Ư�ײ�ƥ����������Ҫ��ȥ��ͨ������Na2SO3�������ȼ��������Ⱥ�IJ���ΪNa2SO4���ȱ�Ϊ��1�ۡ����ѱ����в�����Cl2��ȫת������ҪNa2SO3������Ϊ_________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com