
(2013���������ۣ�27)�ú���Al2O3��SiO2������FeO��xFe2O3�������Ʊ�Al2(SO4)3��18H2O��������������(���ֲ�����������)��
��.�������м������ϡH2SO4�����ˣ�
��.����Һ�м������KMnO4��Һ��������Һ��pHԼΪ3��
��.���ȣ�����������ɫ���������ã��ϲ���Һ���Ϻ�ɫ��
��.����MnSO4���Ϻ�ɫ��ʧ�����ˣ�
��.Ũ�����ᾧ�����룬�õ���Ʒ��
(1)H2SO4�ܽ�Al2O3�����ӷ���ʽ��_________________________________________
________________________________________________________________________��
(2)��MnO ����Fe2�������ӷ���ʽ����������
����Fe2�������ӷ���ʽ����������
 MnO
MnO ��
�� Fe2����
Fe2���� ________===
________=== Mn2����
Mn2���� Fe3����
Fe3���� ________��
________��
(3)��֪��
�����������������pH
| Al(OH)3 | Fe(OH)2 | Fe | |
| ��ʼ����ʱ | 3.4 | 6.3 | 1.5 |
| ��ȫ����ʱ | 4.7 | 8.3 | 2.8 |
ע���������ӵ���ʼŨ��Ϊ0.1 mol��L��1
���ݱ������ݽ��Ͳ�����Ŀ��___________________________________________��
(4)��֪��һ�������£�MnO ����Mn2����Ӧ����MnO2��
����Mn2����Ӧ����MnO2��
�����ij����м���ŨHCl�����ȣ���˵�������д���MnO2�������� ________________________________________________________________________��
�ڢ��м���MnSO4��Ŀ����_____________________________________________��
�𰸡�(1)Al2O3��6H��===2Al3����3H2O
(2)5��8��H����5��4��H2O
(3)pHԼΪ3ʱ��Fe2����Al3�������γɳ�������Fe2������ΪFe3������ʹ����ȫ����
(4)�����ɻ���ɫ���塡�ڳ�ȥ������MnO
������(1)H2SO4�ܽ�Al2O3�����ӷ���ʽΪAl2O3��6H��===2Al3����3H2O���ڲ������У�FeO��xFe2O3Ҳͬʱ���ܽ⣬��Һ�к���Al3����Fe2����Fe3���ȡ�
(2)�����������Һ�л��й���H2SO4���ʷ�Ӧ�����������½��У���������ԭ��Ӧ�е�ʧ�����غ��֪MnO ��Fe2���Ļ�ѧ������֮��Ϊ1��5��Ȼ��۲���ƽ����MnO
��Fe2���Ļ�ѧ������֮��Ϊ1��5��Ȼ��۲���ƽ����MnO ��5Fe2����8H��===Mn2����5Fe3����4H2O��
��5Fe2����8H��===Mn2����5Fe3����4H2O��
(3)������У��������KMnO4��ҺĿ���ǰ�Fe2���������׳�ȥ��Fe3��������pHԼΪ3���ɱ������ݷ�������֪������ʹFe3����ȫ������Al3�������������Ӷ��ﵽ����Fe3����Al3����Ŀ�ġ�
(4)������Cl2��ʵ�����Ʒ�����������ʵ����Ƶ���ͼ����ͨ���Ƿ����Cl2���жϳ���������MnO2���ڲ������м���MnSO4���Ϻ�ɫ��ʧ��˵��������MnO �����MnSO4����ת���ɳ�����Ȼ����˳�ȥMnO
�����MnSO4����ת���ɳ�����Ȼ����˳�ȥMnO ����֤��Ʒ�Ĵ��ȡ�
����֤��Ʒ�Ĵ��ȡ�


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ԫ��X��Ԫ��Y�����ڱ���λ�����ڵ��������ڣ�X��Y��ԭ�Ӻ����������֮��Ϊ19��Yԭ�Ӻ�����������X��3��������������ȷ���ǣ� ��
A��X��Y�������ʻ��õ�Ԫ�أ�����Ȼ���в����Ի���̬����
B��X��Y�γɵĻ�����Ļ�ѧʽΪY2X3
C��XԪ�ص����Ϊ+6��
D��Y���û������⣬�ų�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��CuSO4��Һ����μ���KI��Һ���������۲쵽������ɫ����CuI����Һ��Ϊ��ɫ������Ӧ��Ļ�����в���ͨ��SO2���壬��Һ�����ɫ�����з�����ȷ����
A���μ�KI��Һʱ��ת��2 mol����ʱ����1 mol��ɫ����
B��ͨ��SO2����Һ�����ɫ��������SO2��Ư����
C��ͨ��SO2ʱ��SO2��I2��Ӧ��I2����ԭ��
D������ʵ�������£����ʵ������ԣ�Cu2+>I2>SO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��P��CuSO4��H2O�D��Cu3P��H3PO4��H2SO4(δ��ƽ)�ķ�Ӧ�У�7.5 mol CuSO4������P�����ʵ���Ϊ________mol������1 mol Cu3Pʱ���μӷ�Ӧ��P�����ʵ���Ϊ________mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(2013���Ϻ���22)һ������CuS��Cu2S�Ļ����Ͷ��������HNO3�У��ռ�������V L(��״��)����Ӧ�����Һ��(����Cu2����SO )��������NaOH��������ɫ���������ˣ�ϴ�ӣ����գ��õ�CuO 12.0 g������������ΪNO��NO2�Ļ����������Ϊ1��1����V����Ϊ (����)
)��������NaOH��������ɫ���������ˣ�ϴ�ӣ����գ��õ�CuO 12.0 g������������ΪNO��NO2�Ļ����������Ϊ1��1����V����Ϊ (����)
A��9.0 L B��13.5 L
C��15.7 L D ��16.8 L
��16.8 L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ӿ���ѧ���ϲ�ã�һ����������Ȼ��������·�Ӧ��14CuSO4��5FeS2��12H2O===7X��5FeSO4��12H2SO4������˵����ȷ���� (����)
A��X�Ļ�ѧʽΪCuS�������������������ǻ�ԭ����
B��5 mol FeS2������Ӧ����10 mol����ת��
C�������е�SO ��һ��������������
��һ��������������
D��FeS2ֻ����ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ�ϴ�4J29�Ͻ�(�������Ͻ�)��������ȡ�ܺ�����һ�����������ܽ�Ͻ�ʹ֮��Ϊ
Fe2����Co2����Ni2�����ٰ�Fe2������ΪFe3�����Ӷ�ʹFe3��ת��Ϊij�ֳ����������ﵽ��Ni2����Co2�������Ŀ�ġ�������ҪʹFe2������ΪFe3��������ʹCo2����Ni2�����������Լ���NaClO��NaClO3(��������H2SO4)��Һ����Ӧ�IJ��ֻ�ѧ����ʽ����(AΪ��ԭ��)��
NaClO��A��B�D��NaCl��C��H2O
NaClO3��A��B�D��NaCl��C��H2O
(1)��������ϻ�ѧ����ʽ��_______________________________________________��
________________________________________________________________________��
ʵ�������в���NaClO3������Fe2���ȽϺ��㣬��������
________________________________________________________________________��
(2)��ƽ�������ӷ���ʽ�����ش����⡣
 Fe(OH)3��
Fe(OH)3�� ClO����
ClO���� OH��===
OH��=== FeO
FeO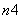 ��
�� Cl����
Cl���� H2O
H2O
(3)��֪��3.21 g Fe(OH)3�μӷ�Ӧ����ת����5.418��1022�����ӣ���n��________��
(4)��������(2)(3)���Ʋ�FeO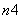 ����������Щ���ʷ�Ӧ________(ֻ�����)��
����������Щ���ʷ�Ӧ________(ֻ�����)��
A��Cl2 B��SO2 C��H2S D��O2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������ܱ������У�2A(g)��2B(g) 3C(g)��D(g)�ﵽƽ��״̬�ı�־�ǣ�
3C(g)��D(g)�ﵽƽ��״̬�ı�־�ǣ�
A����λʱ��������2n mol A��ͬʱ����n mol D
B��������ѹǿ����ʱ����仯
C����λʱ��������n mol B��ͬʱ����1.5n mol C
D�������ڻ�������ܶȲ���ʱ����仯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ܱ������ڣ����淴Ӧ�� ���ﵽƽ��״̬�ı�־�ǣ�
���ﵽƽ��״̬�ı�־�ǣ�
��1����λʱ�������� ��ͬʱ����
��ͬʱ����
��2����λʱ�������� ��ͬʱ����
��ͬʱ����
��3���� �����ʵ���Ũ�ȱ仯��ʾ��Ӧ���ʵı�Ϊ2:2:1��״̬
�����ʵ���Ũ�ȱ仯��ʾ��Ӧ���ʵı�Ϊ2:2:1��״̬
��4������������ɫ���ٸı��״̬
��5����������ƽ����Է����������ٸı��״̬
A. ��1����3����4�� B. ��2����3����5��
C. ��1����4����5�� D. ��1����2����3����4����5��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com