
����Ŀ����ͼΪһ��������ˮ����װ�ã���װ�ÿ�����һ�����ォ�л���ˮ�Ļ�ѧ��ֱ��ת��Ϊ���ܣ���ͼ��һ���ö��Ե缫��ⱥ��ʳ��ˮ������Һ�����������ڼס��ҵ�˵����ȷ����
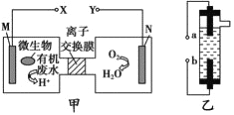
A. װ�����е�b��Ҫ��װ�ü�X������
B. װ������a���ĵ缫��ӦʽΪ:2Cl- -2e-=Cl2��
C. ��N������5.6L(��״����)����ʱ������2NA��H+ͨ�����ӽ���Ĥ
D. ���л���ˮ����Ҫ���������ǣ���װ�ü���M�������ĵ缫��ӦΪ: C6H12O6+6H2O-24e-=6CO2��+24H+
���𰸡�D
������������֪���ͼʾ����Ϊԭ��أ�XΪ������YΪ��������Ϊ�ö��Ե缫��ⱥ��ʳ��ˮ������Һ����������aΪ��������������NaOH��bΪ��������Cl2��������Cl2��NaOH��Ӧ����NaClO��
A�bΪ����������Ӧ��������(Y��)���ӣ���A����B�aΪ�������缫��ӦʽΪ��2H2O+2e-=2OH-+H2����bΪ�������缫��ӦʽΪ��2Cl--2e-=Cl2������B����C���ͼ��N�缫Ϊ�����õ��ӷ�����ԭ��Ӧ���缫��ӦʽΪ��O2+4H++4e-=2H2O����N�缫����5.6L(��״����)����(��0.25mol)ʱ������1mol�����ӣ�����NA��H+ͨ�����ӽ���Ĥ����C����D����л���ˮ����Ҫ���������ǣ���װ�ü���M������C6H12O6ʧ���ӵ�������Ӧ�����ɶ�����̼�����ݵ���غ��ԭ���غ㣬��缫ӦΪ��C6H12O6+6H2O-24e-=6CO2��+24H+����D��ȷ��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͷ����������H��һ�־��п������õĻ����ʵ�����ɷ��㻯����A�Ʊ�H��һ�ֺϳ�·������:
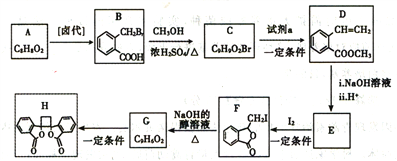
��֪:i.RCH2Br![]() R-CH=CH-R
R-CH=CH-R
ii.2R-CH=CH-R'![]()
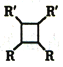 (����R��R'�������������)
(����R��R'�������������)
(1)A�Ļ�ѧ������_____________��C�����������ŵ�����Ϊ_______________��
(2)�Լ�a��____________________��G����H�ķ�Ӧ������_____________________��
(3)��F����G�Ļ�ѧ����ʽ��_______________________________��
(4)D��ͬ���칹���У���ͬʱ�������������Ĺ���_______��(��������ṹ);
�ٱ�����ֻ������ȡ����
����ʹ������Ȼ�̼��Һ��ɫ
�ۼ��ܷ���������Ӧ���ܷ���ˮ�ⷴӦ��ˮ�����֮һ�����Ȼ�����Һ������ɫ��Ӧ�����к˴Ź�������Ϊ�������л���Ľṹ��ʽΪ_________________________��
(5)���Ҵ�Ϊ��ʼԭ�ϣ������֪��Ϣѡ�ñ�Ҫ�����Լ��ϳ� ��д���ϳ�·��_________________(�ü�ʽ��ʾ�л���ü�ͷ��ʾת����ϵ����ͷ��ע���Լ��ͷ�Ӧ����)��
��д���ϳ�·��_________________(�ü�ʽ��ʾ�л���ü�ͷ��ʾת����ϵ����ͷ��ע���Լ��ͷ�Ӧ����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���¹���ѧ�ҹ�����1902 �꿪ʼ�о��ɵ���������ֱ�Ӻϳɰ�����Ӧԭ��Ϊ��N2(g)+3H2(g)![]() 2NH3(g) ��H=-92.4kJmol-1
2NH3(g) ��H=-92.4kJmol-1
��1���ں��º��������£���Ӧƽ����ϵ�г��뵪�����ﵽ��ƽ��ʱ��c(H2)��_________(����������������С�������������������ж�������ͬ)��c(N2)��c3(H2)��___________��
��2����ҵ�Ͽ���CH4��ˮ������������CH4(g)+H2O(g)![]() CO(g)+3H2(g)����200��ʱ2L���ܱ������У���1molCH4��1mol H2O(g)��ϣ���ƽ��ʱCH4 ��ת����Ϊ80%����200��ʱ�÷�Ӧ��ƽ�ⳣ��K=______________������һλС������
CO(g)+3H2(g)����200��ʱ2L���ܱ������У���1molCH4��1mol H2O(g)��ϣ���ƽ��ʱCH4 ��ת����Ϊ80%����200��ʱ�÷�Ӧ��ƽ�ⳣ��K=______________������һλС������
��3����ͼΪ�ϳɰ���Ӧ�ڲ�ͬ�¶Ⱥ�ѹǿ��ʹ����ͬ���������£���ʼʱ�����������������Ϊ1:3 ʱ��ƽ�������а���������������ֱ���vA(NH3)��vB(NH3)��ʾ�ӷ�Ӧ��ʼ����ƽ��״̬A��B ʱ�Ļ�ѧ��Ӧ���ʣ���vA(NH3)____ ( ����>����<������=��)vB(NH3)��

��4����ҵ�������ݳ��İ�����ϡ�������ա���ǡ������NH4HSO4������Һ�и����ӵ����ʵ���Ũ���ɴ�С��˳����______________________��
��5��H2NCOONH4�ǹ�ҵ�ϳ����ص��м����÷�Ӧ�������仯��ͼ��ʾ����CO2�Ͱ��ϳ����ص��Ȼ�ѧ����ʽΪ_________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������������Ҫ��ҽҩ�м��壬��A��BΪԭ�Ϻϳɱ�����������F·�����£�
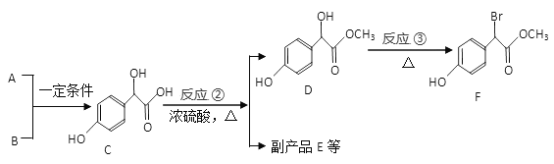
��1�� A �ķ���ʽΪC2H2O3��1molA �����ĺ���2.5 molCu(OH)2 ��������Һ��д����Ӧ���Ļ�ѧ����ʽ_______________________________��
��2��������C ��������������Ϊ______________����һ��������C ������NaOH ��Һ��Ӧ��1molC �������NaOH �����ʵ���Ϊ_________________mol��
��3����Ӧ���ķ�Ӧ����Ϊ___________�� ��д����Ӧ���Ļ�ѧ����ʽ_____________________________��
��4��E ����2 ������C���ɵĺ���3 ����Ԫ���Ļ����E �ķ���ʽΪ____________________��
��5������������F ������ͬ���칹��(�����������칹) ��__________�֣����к˴Ź��������������Ľṹ��ʽΪ____________________��
������һԪ��������� ��������ֻ��2 ��ȡ���� ����FeCl3 ��Һ����ɫ
��6�����������ϳ�·�ߡ��Ա�����Ϊԭ�������Լ���ѡ������Ʊ�A �ĺϳ�·�ߡ���֪RCH2COOH ![]() RCH(Cl)COOH______________________
RCH(Cl)COOH______________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������������뻯ѧ��ص����ݱ���
����ͼ��ij��ͯ��Ԫ����챨�浥�IJ������ݣ�ijҽ�ƻ����ٴ����������浥
������Ŀ | ����� | ��λ | �ο���Χ | |
1 | �Zn�� | 115.92 | ��mol/L | 66~120 |
2 | ����Fe�� | 6.95 | mmol/L | 7.52~11.82 |
3 | �ƣ�Ca�� | 1.68 | mmol/L | 1.55~2.10 |
���� |
�����ϱ������ݣ��ش��������⣺
(1)�ö�ͯ__________Ԫ�غ���ƫ�͡�
(2)���浥������mol/L����__________�������������������������Ũ�������ĵ�λ��
(3)����ά����C��ʹʳ���е�Fe3+ת��ΪFe2+�����������������ά����C��__________��������������������ԭ��������
��������Ϊ���ӳ��ʻ���������ͨ�����ڻ�ƿ�м����ʻ����ʼ����±���0.5Lij���ʻ����ʼ��к��еijɷּ��������Ķ���ش��������⣺
�ɷ� | ����(g) | Ħ������(g/mol) |
�����ǣ�C12H22O11�� | 25.00 | 342 |
0.25 | 174 | |
�۸�����أ�KMnO4�� | 0.25 | 158 |
�ܰ�˾ƥ�֣�C9H8O4�� | 0.17 | 180 |
����������AgNO3�� | 0.02 | 170 |
(4)�����ʻ����ʼ��ɷ��У������ε���__________������ţ���
(5)������500mL���ʻ����ʼ��������²������裺
a���ѳ����õı��ʼ�����С�ձ��У�����������ˮ�ܽ⣻
b����a������ҺС��ת��500mL����ƿ�У�
c������������ƿ�м�����ˮ��Һ���̶�1cm~2cm�������ý�ͷ�ι�С�ĵμ�����ˮ����Һ��Һ����ʹ���̶������У�
d������������ˮϴ���ձ��Ͳ�����2~3�Σ�ÿ��ϴ��Һ��С��ת������ƿ��������ҡ�ȣ�
e��������ƿ�������������µߵ�ҡ�ȡ�
�ٲ����������ȷ˳��Ϊ������ţ�__________��
��������ƿ��ʹ�÷����У����в�������ȷ����__________��
A��ʹ������ƿǰ������Ƿ�©ˮ
B������ƿ������ˮϴ������Ҫ����
C���Ǻ�ƿ������һֻ�ֵ�ʳָ��סƿ������һֻ�ֵ���ָ��סƿ�ף�������ƿ��ת��ҡ������
(6)д�����ʻ����ʼ���K+�����ʵ���Ũ�ȵ�����ʽc(K+)��__________ mol��L1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
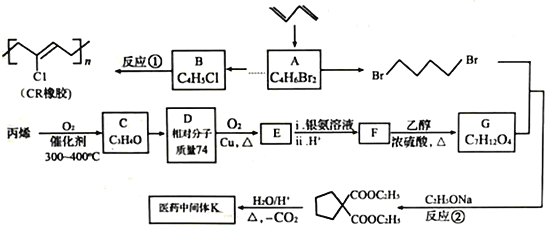
����Ŀ��ʯ���ѽ�����;�㷺����������ʯ���ѽ���Ϊԭ�Ϻϳ�CR��ҽҩ�м���K��·�ߣ�
��֪��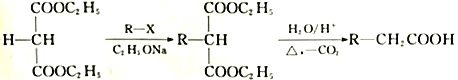
�ش��������⣺
��1��A��˳ʽ�칹��Ľṹ��ʽΪ________________��B�Ļ�ѧ������________________��C��������״�ṹ�������������Ϊ____________________________��
��2��K�Ľṹ��ʽΪ____________����Ӧ�١��ڵķ�Ӧ���ͷֱ�Ϊ_____________��_____________��
��3��D��E�Ľṹ��ʽ�ֱ�Ϊ_____________��_____________��
��4��F���Ҷ��������ۺϷ�Ӧ�Ļ�ѧ����ʽΪ_________________________________��
��5��д��ͬʱ��������������ҽҩ�м���K������ͬ���칹��Ľṹ��ʽ��_________________________________________________��
a.��E��Ϊͬϵ�� b.�˴Ź���������ʾΪ3���
��6����֪˫���ϵ���ԭ�Ӻ��ѷ���ȡ����Ӧ����AΪ��ʼԭ�ϣ�ѡ�ñ�Ҫ�����Լ��ϳ�B��д���ϳ�·�ߣ�_______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��̼�������仯����������������ء������Ҫ��ش���������:
��1����CH4����ԭ��������������������������Ⱦ����֪:
��CH4(g)+4NO2(g)= 4NO(g)+CO2(g)+2H2O(g) ��H=-574kJ/mol
��CH4(g)+4NO(g)= 2N2(g)+CO2(g)+2H2O(g) ��H=-l160kJ/mol
��H2O(g)= H2O(l) ��H=-44kJ/mol
д��CH4(g)��NO2(g)��Ӧ����N2(g)��CO2(g)��H2O( 1) ���Ȼ�ѧ����ʽ_________��
��2����֪: ��ӦI: 4NH3(g)+5O2(g) ![]() 4NO(g)+6H2O(g) ��H < 0
4NO(g)+6H2O(g) ��H < 0
��ӦII: 4NH3(g)+3O2(g) ![]() 2N2(g)+6H2O(g) ��H < 0
2N2(g)+6H2O(g) ��H < 0
���ں��º���װ���г���һ������NH3 ��O2,�ڴ����������½��з�ӦI���������й���������ȷ����___(����ĸ���)��
A.����ѹǿ��Kp(��ѹǿ��ʾ�Ļ�ѧƽ�ⳣ��) ����
B.�����������3v��(NH3)=2v��(H2O) ʱ��˵����Ӧ�Ѵ�ƽ��
C.�����¶ȣ�NH3 ��ת��������
D.�ﵽƽ��ʱ��ϵ��ѹǿ���ٱ仯
����������ʱ�ᷢ����������������ӦI��II��Ϊ����ij�����Ը÷�Ӧ��ѡ���ԣ���1L�ܱ������г���1molNH3��2molO2�����й����ʵ�����ϵ��ͼ���ô����ڸ���ʱѡ��Ӧ_____(����I������II��)��
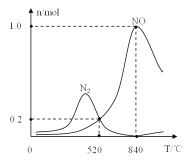
��520��ʱ��4NH3(g)+3O2 (g)![]() 2N2(g)+6H2O(g)��ƽ�ⳣ��K=_______(ֻ���г����ּ���ʽ)�����NH3ת��ΪN2ƽ��ת���ʵĴ�ʩ��______( ����ĸ���)
2N2(g)+6H2O(g)��ƽ�ⳣ��K=_______(ֻ���г����ּ���ʽ)�����NH3ת��ΪN2ƽ��ת���ʵĴ�ʩ��______( ����ĸ���)
A.���ͷ�Ӧ�¶� B.����Ӧ���ɵ�H2O(g)��ʱ�Ƴ�
C.����NH3��O2�ij�ʼͶ�ϱ� D.Ͷ�ϱȲ��䣬���ӷ�Ӧ���Ũ��
��3����֪25��ʱHCN ��H2CO3�ĵ��볣��(Ka.)���±�:
���� | ���볣��(Ka) |
HCN | Ka=5��10-10 |
H2CO3 | Ka1=4.5��10-7��Ka2=4.7�� 10-11 |
25��ʱ�����HCN ��NaCN �Ļ����ҺpH=11,��c(HCN)/c(CN-)=____����NaCN ��Һ��ͨ������CO2��������Ӧ�����ӷ���ʽΪ:__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)��ȥFe(OH)3�����л��е�FeCl3�ķ�����_________________��
(2)��һ�������£�ij��̬������X���ȷֽ�ķ�ӦΪ��2X ![]() A����B����2C������÷�Ӧ�����ɵĻ��������ܶ���ͬ��ͬѹ��H2�ܶȵ�12��������X��Ħ������Ϊ_______��
A����B����2C������÷�Ӧ�����ɵĻ��������ܶ���ͬ��ͬѹ��H2�ܶȵ�12��������X��Ħ������Ϊ_______��
(3)����״���µ�a L HCl��������1 Lˮ�У��õ��������ܶ�Ϊb g/cm3�������������ʵ���Ũ��Ϊ________________��
(4)����ij�¶��±���NaCl��ҺV mL���ܶ�Ϊ��gcm-3�����ʵ���Ũ��ΪC molL-1����¶���NaCl���ܽ��Ϊ_________________����V���ѡ�C��ʾ����
(5)��A��B��C��D ���ֿ������Σ����ǵ���������Ba2+��Ag+��Na+��Cu2+ �е�ijһ�֣���������NO3-��SO42-��Cl-��CO32- ��ijһ�֡�
�����������ηֱ��ܽ���ʢ������ˮ����ֻ�Թ��У�C�ε���Һ����ɫ��
������ٵ���ֻ�Թ��зֱ�����ᣬB��Һ�г���������D��Һ����ɫ��ζ�����ݳ���
���ݢ٢�ʵ����ʵ���ƶ����ǵĻ�ѧʽΪ��
A��____________ C��______________ D��______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ʵ�����ؼ������
(1)19.6 g ������_____mol H2SO4 ��1.7 gNH3 ��ͬ��ͬѹ����_____g H2S ���庬�е���ԭ������ͬ��
(2)�ڱ�״���£�1.6 g ij��̬������ RO2 ���Ϊ 0.56L������������ʵ�����_____��R ���� ��ԭ����Ϊ_____��
(3)��֪ Wg ���� A ���� a �����ӣ���ô�ڱ�״���£�bg ���� A ��ռ������� _____L �����а� ���ӵ������� NA ��ʾ��
(4)��״���£��ܶ�Ϊ 0.75g/L �� NH3 �� CH4 ��ɵĻ�������У�NH3 ���������_______________�� ��������ƽ��Ħ������Ϊ_____�� �����������������ܶ�Ϊ__________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com