
����Ŀ����ͬѧ��Ʋ�����������ʵ�飬��̽����Ȳ����ļӳɷ�Ӧ����ȡһ������ҵ�õ�ʯ��ˮ��Ӧ�������ɵ�����ͨ����������ˮ�У�������Һ��ɫ����֤����Ȳ����ˮ�����˼ӳɷ�Ӧ����ͬѧ�����ڼ�ͬѧ��ʵ���У���ɫ����Һ����������ɫ���ǣ��Ʋ����Ƶ���Ȳ�л����ܺ���������ԭ�Ե��������壬�ɴ�����������ȳ�ȥ֮��������ˮ��Ӧ������ش��������⣺
��1��д����ͬѧʵ���е�������ѧ����ʽ��_______________��____________��
��2������Ϊ����Ƶ�ʵ�鲻����֤�˷�ӦΪ�ӳɷ�Ӧ����������________________��
A��ʹ��ˮ��ɫ�����ʣ�������Ȳ
B��ʹ��ˮ��ɫ�ķ�Ӧ���Ǽӳɷ�Ӧ
C��ʹ��ˮ��ɫ������,δ������Ȳ
D��ʹ��ˮ��ɫ�ķ�Ӧδ���Ǽӳɷ�Ӧ
��3����ͬѧ�Ʋ����Ȳ�бض����е�һ������������_________,����֤�����б���ȫ����ȥ��������ˮ��Ӧ�Ļ�ѧ����ʽ��________________________________________________________��
��4������ѡ����������װ��(����ͼ)�����ظ�ʹ�ã���ʵ����ͬѧ��ʵ�鷽���������ǵı�����뷽��д��װ�������ŵĻ�ѧҩƷ��
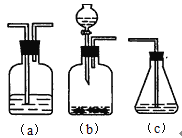
![]()
___��___��___��___
��5��Ϊ��֤��һ��Ӧ�Ǽӳɶ�����ȡ������ͬѧ�������pH��ֽ�����Է�Ӧ����Һ�����ԣ�������___��
���𰸡�CaC2+2H2O��Ca(OH)2+C2H2�� CH��CH+2Br2��CHBr2��CHBr2 C ��D H2S Br2+H2S��S��+2HBr b a CuSO4��Һ CuSO4��Һ ��������ȡ����Ӧ���ض�����HBr����Һ���Խ���������ǿ���ʿ���pH��ֽ��֤
��������
��1����ʯ��ˮ��Ӧ������Ȳ����Ӧ�ķ���ʽΪCaC2+2H2O��Ca(OH)2+C2H2������Ȳ����̼̼������������ˮ�����ӳɷ�Ӧ��ʹ��ˮ��ɫ����Ӧ�Ļ�ѧ����ʽΪCH��CH+2Br2��CHBr2��CHBr2��
��2��A.��ʹ��ˮ��ɫ�����ʲ�һ��������Ȳ��A����B.ʹ��ˮ��ɫ�ķ�ӦҲ��һ�����Ǽӳɷ�Ӧ��B����C.ʹ��ˮ��ɫ������δ������Ȳ��C��ȷ��D.ʹ��ˮ��ɫ�ķ�ӦҲδ���Ǽӳɷ�Ӧ��D��ȷ����ѡCD��
��3����ʯ�к������ʣ���ˮ���ɵ���Ȳ�к���H2S��������л�ԭ�ԣ��ܰ���ˮ��������Ӧ�Ļ�ѧ����ʽΪBr2+H2S��S��+2HBr��
��4��������������ͭ��Һ��Ӧ���ɺ�ɫ������ͭ��������Ҫ��������ͭ��ȥ���⡣Ϊ�����Ƿ����������Ҫ�ٴ�ͨ������ͭ��Һ��
��5�������������ȡ����Ӧ����ض�����HBr����Һ���Խ���������ǿ���ʿ���pH��ֽ��֤��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��п������п�����̵��ң���ZnO 35%���ϣ�������������������(MnO)������ͭ�� ����������Ͳ�����������ʣ���ҵ�ϳ������������ ZnSO4��7H2O����֪ ZnSO4��7H2O ����������ˮ�������ھƾ���ij��ȤС��ʵ����ģ����� ZnSO4��7H2O ���壬�������£�

��ش�
(1)�������� II �еIJ�����ԭ���� �ش��������⣺
�ٽ�ϱ� 1�� 2������ѡ��� pH ���¶ȷֱ���______________________�� ���У� ���Բ��ü���_________________________������ pH ��
��1 pH��ZnSO4.7H2O �����������ȵ�Ӱ��
pH | ZnSO4.7H2O ��������g�� | ��Ʒ��Fe�ĺ���% | ��Ʒ�������ؽ�������% |
1 | 114.32 | 0.750 | 0.059 |
2 | 114.4 | 0.086 | 0.056 |
3 | 113.68 | 0.034 | 0.054 |
4 | 113.60 | 0.010 | 0.050 |
5 | 112.43 | 0.010 | 0.050 |
��2 �¶ȶ�ZnSO4.7H2O �����������ȵ�Ӱ��
�¶� (��) | ZnSO4.7H2O ��������g�� | ��Ʒ��F�ĺ���% | ��Ʒ�������ؽ�������% |
20 | 111.45 | 0.011 | 0.052 |
40 | 112.89 | 0.010 | 0.051 |
60 | 113.30 | 0.010 | 0.050 |
80 | 113.80 | 0.010 | 0.050 |
90 | 114.40 | 0.091 | 0.048 |
������ KMnO4 ��Һ����Һ�е� Fe2+�������������ֳ�����ͬʱ�������ĸ�����������Ե��������Զ��ֽ�����MnO2 ��������д���ڸû����£�KMnO4 ��Һ���� Fe2+�����ӷ�Ӧ����ʽ_________________________________________�� ����ϡ�����������������˲����п��ܺ��� Zn(NO3)2 �⣬�����ܵ�ȱ���ǣ�_________________________��
(2)��������ʵ������У��ش��������⣺
������ B ����Ҫ�ɷ�Ϊ___________________________��
����μ�����ҺB���Ƿ�����Ԫ��_____________________________________��
��д����������C�����ӷ���ʽ__________________________________________��
(3)Ϊ�ⶨ ZnSO4��7H2O ����Ĵ��ȣ����� K4Fe(CN)6 ��Һ���еζ�����Ҫԭ�����£�2K4Fe(CN)6+ 3ZnSO4= K2Zn3[Fe(CN)6]2��+ 3K2SO4
ȷ��ȡ 5.000g ZnSO4��7H2O ���壬������ˮ�ܽⲢ������ 250mL��ȷ��ȡ����Һ 25.00mL����ƿ�У��� 0.0500mol/L K4Fe(CN)6 ��Һ���еζ��������������±���
ʵ����� | �ζ�ǰ����/mL | �ζ������/mL |
1 | 0.10 | 19.92 |
2 | 1.34 | 21.12 |
3 | 0.00 | 20.10 |
�� ZnSO4��7H2O ����Ĵ�����_______________(������������ʾ��������С�������λ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������ͿƼ��������ش����á�Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч����ij��ѧ�о�С���ͬѧ�ֱ��������ͼ�ס�����ʾ��ʵ�顣��ش�������⣺

(1)������ͼ��ͨ���۲�_____________�����ԱȽϵó����ۡ�ͬѧX�۲������֧�Թܲ������ݵĿ������ɴ˵ó�Fe3����Cu2����H2O2�ֽ�Ĵ�Ч���������__________(����������������������)��������___________
(2)������ͼ����ʾ��ʵ��ʱ��������40 mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԡ�ʵ������Ҫ������������_____________________��
(3)����0.10 mol MnO2��ĩ��50 mL H2O2��Һ�У��ڱ�״���·ų�����������ʱ��Ĺ�ϵ��ͼ��ʾ��
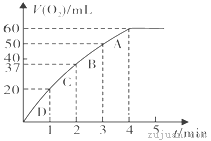
��ʵ��ʱ�ų�������������__________mL��
�ڷų�1/3��������ʱ��Ϊ___________min��
�ۼ���H2O2�ij�ʼ���ʵ���Ũ��_______________�� (�뱣����λ��Ч����)
��A��B��C��D���㷴Ӧ���ʿ�����˳��Ϊ_____��____��____��____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ԫ�����ڱ�����ʽ���ֶ�������ͼ������Ԫ�����ڱ���һ����(1��36��Ԫ��)���Ա���ѧ����Ԫ�����ڱ���˼������Ԫ�����ڱ��������ɣ��Ƴ�ͼ�б�ǵ�11��Ԫ�أ��ش��������⣺
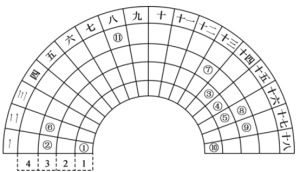
(1)�ݵļ������ӵĽṹʾ��ͼΪ_______����ԭ������Ϊ___________��
(2)�ܵļ��⻯��ĵ���ʽΪ__________________��
(3)��11��Ԫ���У����ʵĻ�ѧ��������õ���__________(�ѧʽ)��
(4)�ࡢ������Ԫ���γɵ�����������ˮ�����У����Խ�ǿ����_______(�ѧʽ)��
(5)����ʱ���۵ĵ����ܺ͢������������ˮ�����Ũ��Һ������Ӧ����ѧ����ʽΪ___________��
(6)����Ԫ�����ڱ��е�λ����_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ü״���ˮ��������Ϊȼ�ϵ���ṩ�������״���ˮ������������Ҫ��Ӧ�ǣ���Ӧi��CH3OH(g)+H2O(g)![]() CO2(g)+3H2(g) ��H1= +49 kJ/mol��ͬʱ�����ڸ���Ӧ����Ӧii��CH3OH(g)
CO2(g)+3H2(g) ��H1= +49 kJ/mol��ͬʱ�����ڸ���Ӧ����Ӧii��CH3OH(g)![]() CO(g)+2H2(g) ��H2= +91 kJ/mol
CO(g)+2H2(g) ��H2= +91 kJ/mol
(1)��Ӧi��ƽ�ⳣ��K�ı���ʽ��_______��
(2)Ϊ̽�������Է�Ӧiƽ���Ӱ�죬X�� Y(Y1��Y2)�ɷֱ����ѹǿ���¶ȡ���ͼ��ʾYһ��ʱ����Ӧi��H2O(g)��ƽ��ת������X�ı仯��ϵ��

�� X��������������_______��
�� �ж�Y1_______Y2(�>����<��)��������_______��
(3)CO��ʹ��Ӧi�Ĵ����ж����о��¶Ⱥ�Ͷ�ϱȶԼ״�ת���ʼ���������CO���ʵ���������Ӱ�죬�����ͼ��ʾ��

��ѡ��250�桢ˮ/�״�Ͷ�ϱ�Ϊ2��Ϊ����������з�Ӧ��ԭ����_______��
��250��ʱCO���ʵ�������ʼ�ո���200��ʱCO���ʵ���������ԭ�������_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ҫ�ش���������
I.����ȼ�ϵ������ʽ�ͼ�ʽ���֣����Ƿŵ�ʱ�ĵ���ܷ�Ӧ��Ϊ2H2+O2 = 2H2O��
(1)����ʱ���������Һ�е�����������_______��(����������������)��
(2)��ʽ����ȼ�ϵ�صĵ������Һ��ϡ���ᣬ�������ĵ缫��ӦΪ________��
(3)��ʽ����ȼ�ϵ�صĵ������Һ��KOH��Һ���为���ĵ缫��ӦΪ_______��
II.ͨ��NO�������ɼ������β����NO�ĺ������乤��ԭ����ͼ��ʾ��O2-���ڹ��������������ƶ���
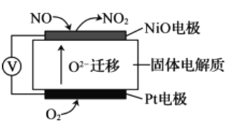
(1)NiO�缫�Ϸ�������________��Ӧ(����������������ԭ��)��
(2)���·�У������Ǵ�_________�缫����(����NiO������Pt��)��
(3)Pt�缫�ĵ缫��ӦΪ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������G��һ��ҩ��ϳɵ��м��壬G��һ�ֺϳ�·�����£�
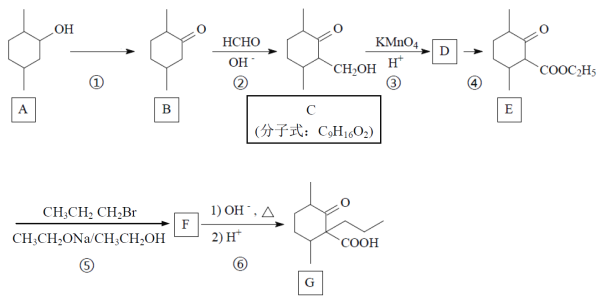
(1)д��A�й����ŵĵ���ʽ��_____________��
(2)д����Ӧ���ͣ�B��C___________��Ӧ��C��D__________��Ӧ��
(3) A��B���跴Ӧ�Լ��ͷ�Ӧ����Ϊ_______________________________��
(4) д��C�ķ�����������ͬ���칹��Ľṹ��ʽ��_________________________��(��д��3��)
����ˮ�⣻���ܷ���������Ӧ������Ԫ���ṹ���һ���ֻ��һ��̼ԭ������ȡ������
(5)д��F�Ľṹ��ʽ_______________________��
(6)����ѧ����֪ʶ��д���ɼױ�(![]() )��
)��![]() Ϊԭ���Ʊ�
Ϊԭ���Ʊ� �ĺϳ�·�ߡ�(���Լ�����)_____________________��
�ĺϳ�·�ߡ�(���Լ�����)_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����б�ʾ��Ӧ��ѧ��Ӧ�����ӷ���ʽ��ȷ���ǣ� ��
A.MnO2��Ũ���ᷴӦ��Cl2��MnO2+4HCl![]() Mn2��+2Cl��+Cl2��+2H2O
Mn2��+2Cl��+Cl2��+2H2O
B.��������ˮ����Al(OH)3���壺Al3��+3H2O =Al(OH)3��+3H��
C.Na2O2����ˮ����O2��Na2O2+H2O =2Na��+2OH��+O2��
D.Ca(HCO3)2��Һ������NaOH��Һ��Ӧ��HCO3��+Ca2��+OH��= CaCO3��+H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵������ȷ����( )
A.��״���£���22.4 LHCl����1 Lˮ�������1 mol/L��ϡ����
B.��200 mL3 mol/L��MgCl2��Һ��100 mL3 mol/L��KCl��Һ��Ϻ���Һ�е�Cl-�����ʵ���Ũ����Ȼ��3 mol/L
C.�õ���(CuSO45H2O)����500 mL5 mol/L��CuSO4��Һ���赨��40 g
D.��0.1 mol NaCl���100 mL��Һ������ȡ��10 mL����ȡ����Һ�����ʵ���Ũ��Ϊ1 mol/L
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com