
��ѧ��һ����ʵ��Ϊ��������Ȼ��ѧ�����л�ѧʵ��ʱҪ��Ϥ��ѧ��ѧʵ���г�����������Ʒ����ȡ��ȷ��ʵ�������ע��ʵ�鰲ȫ��
(1)��������a.��Һ©����b.�Լ�ƿ��c.����ƿ��d.�ζ��ܡ�e.����ƿ��f.��Ͳ��g.������ƽ�У����С�0���̶ȵ���(����ţ���ͬ) �����о���ʹ���¶ȵ��� ��
(2)��ĥɰ�������Ӳ��������ܷ��Ե�һ�ִ������ա������������У�û���õ���ĥɰ�����մ������� ��
a���Թ�
b����Һ©��
c�����ιܵ��Լ�ƿ(��ƿ)
d������ƿ
e����ʽ�ζ���
f����ʽ�ζ���
(3)���в�������ʵ������ʵ����ֵƫ�ߵ���( )
a������Ͳ��ȡ8.5 mLҺ��ʱ������Һ�����
b���к͵ζ��ﵽ�յ�ʱ������Һ�����
c������һ�����ʵ���Ũ�ȵ���Һ����ʱ��������ҺҺ��
(4)��ʢ��Ũ������Լ�ƿ�ı�ǩ��ӡ����ͼ��ʾ�ľ�ʾ��־��˵��Ũ������һ�� ��
(5)��ͬѧ������ͼ��ʾװ���Դ���ʯ��ϡ���ᷴӦ��ȡCO2����ʦָ��������Ҫ̫���ϡ���ᣬ����˷ѣ���ͬѧѡ����ʵ������õ�һ������������װ���ϣ������������⡣����Ѹ���������ͼ�к��ʵ�λ�á�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����ʵ���������ʵ��Ԥ��ʵ��Ŀ�Ļ����ý���һ�µ���( )
| ѡ�� | ʵ���������ʵ | ʵ��Ŀ�Ļ���� |
| A | ij��Һ ð������ ð������ | ˵��ԭ��Һ��һ������CO32- |
| B | SiO2�봿����¿�����CO2 | ˵����������Ա�̼��ǿ |
| C | �⻯����Һ�����Ի�ɫ | ������I-����ԭ��������I2������Һ�� |
| D | ��������Ũ�����н��ݺ���������ˮ��ϴ��Ȼ�����CuSO4��Һ�в���Ӧ | ˵�����������γ���һ�������ȶ�������Ĥ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��6�֣���ͼ��A��B��C��D�dz�������ͨ�����Լ�ƿ����������Լ��������д���ʺ�ʢ�ŵ��Լ�ƿ����������ڣ�
a��Ũ���� b��̼������Һ c����Ƭ d��Ũ���� e������������Һ f����������
| A | B | C | D |
 |  |  |  |
| �� �� | �� �� | �� �� | �� �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Ϊ�˽�����K2SO4��MgSO4��KNO3�����ᴿ�����Ƶô�����KNO3��Һ��ijѧ���������ʵ�鷽����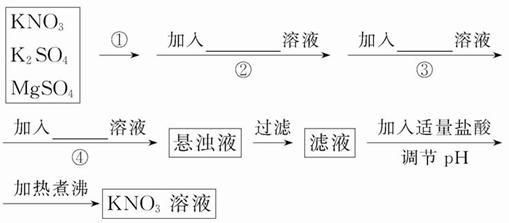
(1)������Ϊ___________________________��
(2)������~�ܼ�����Լ����ο���Ϊ��___________________________________________________��
(3)����ж�SO42-�ѳ�����____________________________��
(4)ʵ������в����Ķ�γ���_______ (���Ҫ������Ҫ��)��ι��ˣ���������_______________________________________________________��
(5)��ͬѧ��ʵ����Ʒ����Ƿ����ܣ���˵�����ɣ�___________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ϣʱ�������Ĵ������������Ի�������˼������в��ij�����Ϊ����ѧ��̽��С�齫һ����������·������õ���70%Cu��25%Al��4%Fe������Au��Pt�Ƚ����Ļ�������Ƴ������Ʊ�����ͭ�����·�ߣ�
�ش��������⣺
�ŵڢٲ�Cu���ᷴӦ�����ӷ���ʽΪ ���õ�����1����Ҫ�ɷ�Ϊ ��
�Ƶڢڲ�����H2O2�������� ��ʹ��H2O2���ŵ��� ������pH��Ŀ����ʹ ���ɳ�����
���õڢ۲�����CuSO4��5H2O�Ʊ���ˮCuSO4�ķ����� ��
��������2��ȡAl2(SO4)3��18H2O��̽��С����������ַ�����
�������ַ����У� ���������У�ԭ���� ��
��ԭ�������ʽǶȿ��ǣ� ������������
��̽��С���õζ����ⶨCuSO4��5H2O(Mr=250)������ȡa g �������100 mL��Һ��ÿ��ȡ20.00 mL�������������Ӻ���c mol/L EDTA(H2Y2��)����Һ�ζ����յ㣬ƽ������EDTA��Һb mL���ζ���Ӧ���£�Cu2++ H2Y2����CuY2��+ 2H+
д������CuSO4��5H2O���������ı���ʽ�أ� ��
���в����ᵼ��CuSO4��5H2O�����ⶨ���ƫ�ߵ��� ��
a��δ������ƿ
b���ζ��յ�ʱ�ζ��ܼ����в�������
c��δ��������EDTA��Ӧ�ĸ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����п��ĩ�㷺Ӧ������Ϳ�ϡ��մɡ�������ҽҩ����������ҵ��Ϊ�ۺ�Ӧ����Դ������ұ��п��п��Ʒ�ӹ���ҵ���յ�п��������ZnO��FeO��Fe2O3��CuO��Al2O3�����ʣ�����ȡ����п��������ͼ��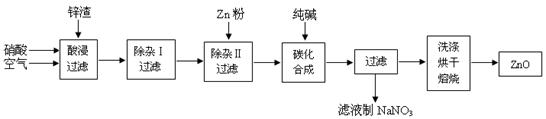
�й��������������ȫ��pH���±���
��1������������У�����п����ɷ�ĩ��ͨ�������ͬʱ����������������� ��
��2���������նദ�漰�����ˡ���ʵ�����й�����Ҫʹ�õIJ����������ձ��� ��
��3�����ڡ����Ӣ��У���������KMnO4��Һ����Ŀ���� ��KMnO4�Ǹ÷�Ӧ�� ������������ԭ����������Һ��pH����4��Ŀ���� ��
���ڡ�����II���У�����п�۵�Ŀ���� ��
��4���ڡ�̼���ϳɡ��У��������м�ʽ̼��п��Zn2(OH)2CO3�ݺ�CO2�ȣ���д���÷�Ӧ�Ļ�ѧ����ʽ ��
��5���������У����˷�������϶࣬�����Ե�ȱ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ʯ�к��е���Ҫ��������������������ij��ѧ��ȤС���ô���ʯΪԭ����ȡ��ȫ��ɱ�����������Ƶ���Ҫ���̣�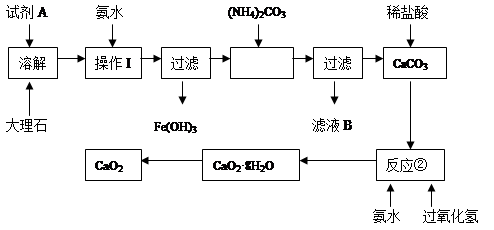
��ش��������⣺
��1���Լ�A�������� ��
��2������I��Ŀ���� ��
��3����ʵ����Ҫ���ʹ�ù��ˣ������С�һ���������͡��������������С����͡�ָ ��
��4��д����Ӧ��������CaO2��8H2O�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�����г����⣬����������Ԫ�أ���̼Ԫ�غ���Ԫ�ء�����̼��Ҫ��̼��������̬���ڣ���ʹ�������ܼ�Ӳ���࣬������������;����һ���������ֵ�ԭ�ϡ�ij��ȤС����ư���ͼ��ʾ��ʵ��װ�ã��ⶨ�����еĺ�̼����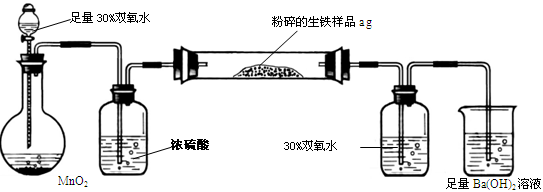
A B C D E
��ش��������⣺
��1���������������к�Ԫ�أ���ʹ���������ȴ��ԡ���Ԫ�������������п��ܴ��ڵļ�̬��
| A����2���� | B��0�� �� | C��+4���� | D��+6 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com