
���������������������ˮ��ҵ��ˮ����������������������Ͽ졣ʵ���ҿ��ö�������Ϊ��Ҫԭ���Ʊ�������ء��䲿���������£�
��1���ڢٲ��в��������������ô�������ԭ���ǣ��û�ѧ����ʽ��ʾ��_______________________________________________________________��
��2��KOH��KClO3��MnO2���۷�Ӧ����ī��ɫK2MnO4�Ļ�ѧ����ʽΪ________________________________________________________________��
��3���ڢܲ�ͨ��CO2������ʹMnO42-������Ӧ������MnO4-��MnO2����K2MnO4��ȫ��Ӧʱ��ת��ΪKMnO4�İٷ���ԼΪ____________________����ȷ��0.1%����
��4���ڢݲ����ȹ��˵�Ŀ����________________________________��
��5���ڢ�����Ũ����Һ����ϸС��������ʱ��ֹͣ���ȣ���ȴ�ᾧ��___________��ϴ�ӡ������������У��¶Ȳ��˹��ߣ���Ϊ_________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ǻ��������ĺ��ģ���������Ļ�������������ô����ա�
�����dz��ô�����ѡ��Ӧ���еķ�����ͼ��ʾΪһ��������1mol CH3OH��O2������Ӧʱ������CO��CO2��HCHO�������仯ͼ[��Ӧ��O2(g)��������H2O(g)��ȥ]��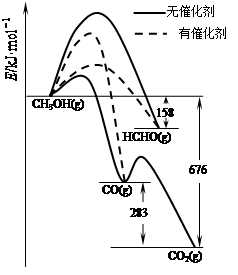
�����д��������£�CH3OH��O2��Ӧ��Ҫ���� ���CO��CO2��HCHO������
��2HCHO(g)��O2(g)=2CO(g)��2H2O(g) ��H= ��
����ϡ������£�HCHO����ͨ����Ӧ���ɷ���ʽΪC3H6O3�Ļ�״���ۼ�ȩ���ӣ��������ͬ��ԭ�ӵĻ�ѧ��������ͬ��д�����ۼ�ȩ�Ľṹ��ʽ�� ��
�ܼ״���ȡ��ȩ����Ag������������AgCl��Ӱ��Ag�����Ļ��ԣ��ð�ˮ�����ܽ��ȥ���е�AgCl��д���÷�Ӧ�����ӷ���ʽ�� ��
��һ����ͭ�����������������������̣�
����ͭ����������ɹ�ҵβ����SO2�IJ��ִ���������������ӦΪ��
2SO2��2n Cu��(n��1)O2��(2��2 n) H2O="2n" CuSO4��(2��2n) H2SO4
�ӻ��������ĽǶȿ��������������Ϊ ��ÿ���ձ�״����11.2L SO2����SO2��ԭ��O2������Ϊ g��
��������ͼ��ʾ�绯ѧװ��������һ����SO2�������Cu��������д��װ������������Ӧ�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1�����Ϸ�����(��Ҫ�ɷ�V2O5)��ϡ���ᡢ���������Һ��ϣ���ַ�Ӧ��������Һ�����ԣ���VO2����K����SO42-�ȡ�д���÷�Ӧ�Ļ�ѧ����ʽ_________________________________��
��2��������������Һ�м���KClO3��Һ����ַ�Ӧ����Һ����������VO2+��Cl����д������ƽ�÷�Ӧ�����ӷ���ʽ�����������ת�Ƶ���Ŀ�ͷ���______________________��
��3����20.00 mL��0.1 mol��L��1 VO2+��Һ�У�����0.195 gп�ۣ�ǡ����ɷ�Ӧ����ԭ���������______________________________________________________________��
a��V b��V2�� c��VO2+ d��VO2��
��4����֪V2O5�ܺ����ᷴӦ����������VO2��������дһ�����ӷ�Ӧ����ʽ��˵����ԭ�ԣ�SO32-��Cl����VO2��__________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(13��)�������Ļ������ڹ�ҵ�������ճ������ж��й㷺����;��
��1���ڶ������У�������������������Ӧ�ų����������и�ֽ�÷�Ӧ�Ļ�ѧ����ʽΪ�ߣߡ�
��2����֪��2Fe2O3(s)��3C(s)��3CO2(g)��4Fe(s) ��H��+468.2 kJ��mol-1
C(s)+O2(g)��CO2(g) ��H="-393.5" kJ��mol-1��
��Fe(s)��O2 (g)��Ӧ����Fe2 O3 (s)���Ȼ�ѧ����ʽΪ��_____________________��
��3������KMnO4��Һ�ζ�Fe2+��Ũ�ȣ���Ӧ�����ӷ���ʽ���£�5Fe2����MnO4����8H����5Fe3����Mn2����4H2O
��KMnO4��ҺӦʢ���ڣߣߣߣߣߵζ����У�
���жϴﵽ�ζ��յ�������ǣߣߣߣߣߣ�
���������ữ��0.020 00 mol��L-1��KMnO4��Һ�ζ�ijFeSO4��Һ���յ㣬ʵ�����ݼ�¼���±���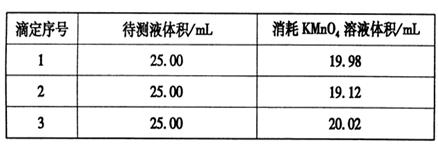
��������ݲ����㣬��FeSO4��Һ�����ʵ���Ũ��Ϊ�ߣߣߣߣߡ�
��4���������ײ���ZnFe2Ox�������ڳ�ȥ��ҵ�����е�ijЩ�������ȡ�²��Ϻͳ�ȥ������ת����ϵ����ͼ��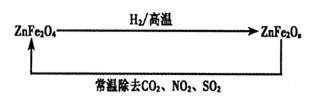
����֪ZnFe2O4��H2��Ӧ�����ʵ���֮��Ϊ2:1����ZnFe2Ox��x=�ߣߣߣߣߣ�
����ZnFe2Ox��ȥSO2�Ĺ����У��������ǣߣߣߣߣߡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������Ч����SO2�Կ�������Ⱦ��
(1)��ú�м���ʯ��ʯ�ɼ���ȼ�ղ�����SO2�ĺ������÷�Ӧ�Ļ�ѧ����ʽ��
_______________________________��
(2)��ˮ�������ԣ���Ҫ����Na����K����Ca2����Mg2����Cl����SO42����Br����HCO3���ȡ���SO2�����������ú�ˮ�����乤��������ͼ��ʾ��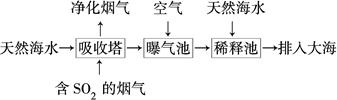
������������ͨ�������Ŀ����_____________________________________��
��ͨ��������������к�ˮ����Ȼ��ˮ��ȣ�Ũ�������Բ�ͬ��������________��
a��Cl�������� b��SO42�������� c��Br�������� d��HCO3��
(3)��NaOH��Һ���������е�SO2�������õ�Na2SO3��Һ���е�⣬�ɵõ�NaOH��ͬʱ�õ�H2SO4����ԭ����ͼ��ʾ(�缫����Ϊʯī)��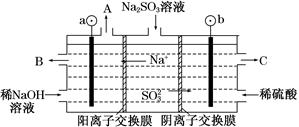
��ͼ��a��Ҫ���ӵ�Դ��________(���������)����C��������������________��
��SO32���ŵ�ĵ缫��ӦʽΪ____________________________��
�۵�����������������������ǿ����ƽ���ƶ���ԭ������ԭ��
__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1���뽫�����������ʣ�KBr��Br2��I2��KI��K2SO4�ֱ��������к����ϣ����һ��δ��ƽ�Ļ�ѧ����ʽ��
KBrO3��________��H2SO4�D��________��________��________��________��H2O��
��2������û�ѧ����ʽ��I2��KBr�Ļ�ѧ�������ֱ���8��1����
��Br2�Ļ�ѧ��������________��
���뽫��Ӧ��Ļ�ѧʽ����ƽ��Ļ�ѧ����������������Ӧ��λ���У�
________KBrO3��________��________H2SO4�D��������
����ת��10 mol���ӣ���Ӧ������I2�����ʵ���Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij̽��С�齫һ�����ӷ���������õ���Cu��Al��Fe������Au��Pt�Ƚ����Ļ�������������Ʊ�����ͭ�������ˮ�Ȼ����ķ�����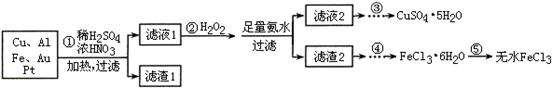
��֪��Cu2+ + 4NH3��H2O��[Cu(NH3)4]2+ + 4H2O
��ش��������⣺
��1�������Cu���ᷴӦ�����ӷ���ʽΪ ��
��2������ڼ�H2O2�������� ������2Ϊ(�ѧʽ) ��
��3������ݲ���ֱ�Ӽ�����ˮ�������� ��
��4������Һ1��Cu2+��Ũ��Ϊ0��02mol��L-1����������ͭ��ʼ����ʱ��pH =
(��֪��Ksp[Cu(OH)2]��2��0��10-20)��
��5����֪��2Cu2+��4I-�� 2CuI����I2 I2��2S2O32-�� 2I-��S4O62-
ijͬѧΪ�˲ⶨCuSO4��5H2O��Ʒ�����������ɰ����·�����ȡ3��00g��Ʒ����ˮ�ܽ����������KI��Һ����ַ�Ӧ����ˡ�ϴ�ӣ�����Һϡ����250mL��ȡ50mL���������Һ��ָʾ������0��080 mol��L-1 Na2S2O3����Һ�ζ����ﵽ�ζ��յ�������� ��
�Ĵ�ƽ��ʵ���ȥNa2S2O3����Һ�������£�
| ʵ����� | 1 | 2 | 3 | 4 |
| ����Na2S2O3����Һ(mL) | 25��00 | 25��02 | 26��20 | 24��98 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧ�ڻ�������������ʮ����Ҫ�����á������������͵绯ѧ���ⷨ����������ˮ�������ε���Ⱦ��
��1�������������У�H2�ܽ�NO3-��ԭΪN2��25��ʱ����Ӧ����l0 min����Һ��pH��7��Ϊl 2��
��N2�ĽṹʽΪ
��������Ӧ�����ӷ���ʽΪ ����ƽ����Ӧ����v(NO3-)Ϊ mol��L-1��min-1��
�ۻ�ԭ�����п������м����NO2-��д��2�ִٽ�NO2-ˮ��ķ��� ��
��2���绯ѧ����NO3-��ԭ������ͼ��ʾ��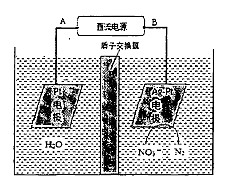
�ٵ�Դ����Ϊ (�A����B��)��������ӦʽΪ��
������������ת����2 mol���ӣ���Ĥ������Һ�������仯��(��m��-��m��)Ϊ g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������ԭ��Ӧ�У�����ת�Ƶı�ʾ����ͨ���õ����ŷ���˫���ŷ����Է��������ֱ�ʾ����������������ա�
��1���굥���ţ�4�֣�
KIO3��6HI��KI��3I2��3H2O���������뻹ԭ�������ʵ���֮�ȣ� ��
��2����ƽ��ѧ����ʽ����˫���ţ�5�֣�
Fe + HNO3(ϡ)�� Fe(NO3)2+ NO��+ H2O��Ӧ�У��������뻹ԭ�������ʵ���֮�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com