
ЎѕМвДїЎїКµСйКТУГТТґјУлЕЁБтЛб№ІИИЦЖИЎТТП©Ј¬іЈТтОВ¶И№эёЯЙъіЙЙЩБїSO2Ді»ЇС§РЛИ¤РЎЧйЙијЖБЛИзНјЛщКѕКµСйЈ¬ТФСйЦ¤ЙПКц»мєПЖшМеЦРКЗ·сє¬УРТТП©єНSO2ЎЈНкіЙПВБРМоїХЈє
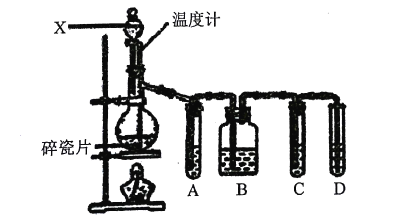
ЈЁ1Ј©Ч°ЦГЦРЛйґЙЖ¬µДЧчУГКЗЈє___ЎЈ
ЈЁ2Ј©ТЗЖчXµДГыіЖКЗ___ЎЈ
ЈЁ3Ј©РґіцЙъіЙТТП©µД·ґУ¦·ЅіМКЅЈє___Ј¬ёГ·ґУ¦µД·ґУ¦АаРНОЄЈє___ЎЈ
ЈЁ4Ј©КФ№ЬAЎўBЎўCЎўDЦРїЙКў·ЕµДКФјБКЗ(ґУПВБРКФјБЦРСЎіцЈ¬КФјБїЙЦШёґК№УГЈ¬МоРтєЕ)Јє
ўЩЖ·ємИЬТє ўЪNaOHИЬТє ўЫдеЛ® ўЬПЎБтЛб
A___Ј»B___Ј»C___Ј»D___ЎЈ
ЈЁ5Ј©И·Ц¤є¬УРТТП©µДПЦПуКЗ___ЎЈ
ЈЁ6Ј©ИфГ»УРКФ№ЬAЎўBЎўCЈ¬ФтФЪКФ№ЬDЦРЛщ·ўЙъ·ґУ¦µД»ЇС§·ЅіМКЅОЄЈє___ЎЈ
Ўѕґр°ёЎї·АЦ№±©·Р ·ЦТєВ©¶· CH3CH2OH![]() CH2=CH2Ўь+H2O ПыИҐ ўЩ ўЪ ўЩ ўЫ CЦРИЬТєІ»НКЙ«Ј¬DЦРИЬТєНКЙ« CH2=CH2+Br2ЎъCH2BrCH2BrЎўSO2+Br2+2H2O=H2SO4+2HBr
CH2=CH2Ўь+H2O ПыИҐ ўЩ ўЪ ўЩ ўЫ CЦРИЬТєІ»НКЙ«Ј¬DЦРИЬТєНКЙ« CH2=CH2+Br2ЎъCH2BrCH2BrЎўSO2+Br2+2H2O=H2SO4+2HBr
ЎѕЅвОцЎї
ЈЁ1Ј©КµСйКТЦЖ±ёТТП©ЛщУГµДФБПОЄТТґјЈ¬ЕЁБтЛбЧчґЯ»ЇјБЎўНСЛ®јБЈ¬·ґУ¦МхјюКЗјУИИµЅ170ЎжЈ¬ТтТТґјµД·РµгµНЈ¬ТЧ±©·РЈ¬ЛщТФјУЛйґЙЖ¬Ј»
ЈЁ2Ј©ёщѕЭТЗЖчµДРОЧґЅб№№РФЦКУГНѕЅшРРЅвґрЈ»
ЈЁ3Ј©КµСйКТАыУГТТґјФЪЕЁБтЛбµДґЯ»ЇЧчУГПВ·ўЙъ·ЦЧУДЪНСЛ®ЦЖИЎТТП©Ј»
ЈЁ4Ј©¶аЦЦІъОпРијмСйК±Ј¬У¦їјВЗПИєуЛіРтЈ»
ЈЁ5Ј©CЦРОЮ¶юСх»ЇБтЈ¬DЦРУлдеЛ®ЧчУГµДОЄТТП©Ј»
ЈЁ6Ј©деєН¶юСх»ЇБтј°ТТП©ѕщДЬ·ґУ¦ЎЈ
ЈЁ1Ј©КµСйКТЦЖ±ёТТП©ЛщУГµДФБПОЄТТґјЈ¬ЕЁБтЛбЧчґЯ»ЇјБЎўНСЛ®јБЈ¬·ґУ¦МхјюКЗјУИИµЅ170ЎжЈ¬ТтТТґјµД·РµгµНЈ¬ТЧ±©·РЈ¬ЛщТФјУЛйґЙЖ¬·АЦ№±©·РЈ»
ЈЁ2Ј©Ч°ЦГЦРXТЗЖчїЙНЁ№э»оИыїШЦЖµОјУТєМеµДЛЩ¶ИАґїШЦЖ·ґУ¦µДїмВэЈ¬ЛщТФЛьµДГыіЖКЗ·ЦТєВ©¶·Ј»
ЈЁ3Ј©КµСйКТАыУГТТґјФЪЕЁБтЛбµДґЯ»ЇЧчУГПВ·ўЙъ·ЦЧУДЪНСЛ®ЦЖИЎТТП©Ј¬ТТґј·ўЙъБЛПыИҐ·ґУ¦Ј¬·ґУ¦·ЅіМКЅОЄЈєCH3CH2OH![]() CH2=CH2Ўь+H2OЈ»
CH2=CH2Ўь+H2OЈ»
ЈЁ4Ј©јмСй¶юСх»ЇБтУГЖ·ємИЬТєЈ¬јмСйТТП©УГёЯГМЛбјШЛбРФИЬТєЈ¬ТТП©єН¶юСх»ЇБт¶јДЬК№ёЯГМЛбјШЛбРФИЬТєНКЙ«Ј¬ЛщТФПИјмСй¶юСх»ЇБтЈ¬И»єујмСйТТП©Ј¬Н¬ФЪјмСйТТП©Ц®З°УГNaOHИЬТєіэѕЎSO2Ј¬ФЩНЁ№эЖ·ємИЬТєІ»НКЙ«И·ИПSO2ТСіэёЙѕ»Ј¬ЧоєуУГёЯГМЛбјШЛбРФИЬТєНКЙ«јмСйТТП©Ј¬ТтЧ°ЦГAУГАґјмСйSO2Ј¬КФ№ЬЦРЖ·ємИЬТєНКЙ«Ј¬ЛµГчє¬УРSO2Ј¬Ч°ЦГBКФ№ЬЧ°УРNaOHИЬТєіэИҐSO2Ј¬Ч°ЦГCКФ№ЬНЁ№эЖ·ємИЬТєІ»НКЙ«И·ИПSO2ТСіэёЙѕ»Ј¬Ч°ЦГD НЁ№эдеЛ®НКЙ«јмСйТТП©Ј¬
№Кґр°ёОЄЈєўЩЈ»ўЪЈ»ўЩЈ»ўЫЈ»
ЈЁ5Ј©ТтCЦРОЮ¶юСх»ЇБтЈ¬DЦРУлдеЛ®ЧчУГµДОЄТТП©Ј¬ЛщТФИ·Ц¤є¬УРТТП©µДПЦПуКЗЧ°ЦГCЦРµДЖ·ємИЬТєІ»НКЙ«Ј¬DЦРµДдеЛ®НКЙ«Ј¬
№Кґр°ёОЄЈєЧ°ЦГCЦРµДЖ·ємИЬТєІ»НКЙ«Ј¬DЦРµДдеЛ®НКЙ«Ј»
ЈЁ6Ј©ИфГ»УРКФ№ЬAЎўBЎўCЈ¬ФтФЪКФ№ЬDЦРє¬УРµД¶юСх»ЇБтЎўТТП©ѕщДЬУлде·ґУ¦¶шК№ЖдНКЙ«Ј¬Лщ·ўЙъ·ґУ¦µД»ЇС§·ЅіМКЅОЄЈєCH2=CH2+Br2ЎъCH2BrCH2BrЎўSO2+Br2+2H2O=H2SO4+2HBrЎЈ


 МмМмПтЙПТ»±ѕєГѕнПµБРґр°ё
МмМмПтЙПТ»±ѕєГѕнПµБРґр°ё РЎС§Йъ10·ЦЦУУ¦УГМвПµБРґр°ё
РЎС§Йъ10·ЦЦУУ¦УГМвПµБРґр°ё
| Дкј¶ | ёЯЦРїОіМ | Дкј¶ | іхЦРїОіМ |
| ёЯТ» | ёЯТ»Гв·СїОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СїОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СїОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СїОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СїОіМНЖјцЈЎ | іхИэ | іхИэГв·СїОіМНЖјцЈЎ |
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїБЪјЧ»щ±ЅјЧЛбЦчТЄУГУЪЕ©Т©ЎўТЅТ©ј°УР»ъ»Ї№¤ФБПµДєПіЙЈ¬ЖдЅб№№јтКЅОЄ Ј¬ПВБР№ШУЪёГОпЦКµДЛµ·ЁХэИ·µДКЗЈЁЎЎЎЎЈ©ЎЈ
Ј¬ПВБР№ШУЪёГОпЦКµДЛµ·ЁХэИ·µДКЗЈЁЎЎЎЎЈ©ЎЈ
A.ёГОпЦКДЬУлдеЛ®ЙъіЙ°ЧЙ«іБµн
B.ёГОпЦКє¬±Ѕ»·µДН¬·ЦТм№№МеЦРДЬЛ®ЅвЗТє¬УРјЧ»щµД№І5ЦЦ
C.1molёГОпЦКЧо¶аДЬУл4molH2ЙъјУіЙ·ґУ¦
D.ёГОпЦКЦРЛщУРФЧУ№ІЖЅГж
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїЈЁ1Ј©ВИЛбКЗТ»ЦЦЗїЛбЈ¬ВИЛбµДЕЁ¶Иі¬№э40%ѕН»бСёЛЩ·ЦЅвЈ¬·ґУ¦µД»ЇС§·ЅіМКЅОЄ8HClO3=3O2ЎьЈ«2Cl2ЎьЈ«4HClO4Ј«2H2OЎЈёщѕЭМвТвНкіЙПВБРРЎМвЈє
ўЩФЪЙПКц±д»Ї№эіМЦРЈ¬·ўЙъ»№Ф·ґУ¦µД№эіМКЗ_____Ўъ____ЈЁМо»ЇС§КЅЈ©ЎЈ
ўЪёГ·ґУ¦µДСх»ЇІъОпКЗ________ЈЁМо»ЇС§КЅЈ©ЎЈ
ЈЁ2Ј©ТСЦЄІв¶ЁГМµДТ»ЦЦ·Ѕ·ЁКЗЈєГМАлЧУЧЄ»ЇОЄёЯГМЛбёщАлЧУЈ¬·ґУ¦МеПµЦРУРHЈ«ЎўMn2Ј«ЎўH2OЎўIO3ЈЎўMnO4ЈЎўIO4ЈЎЈУР№Ш·ґУ¦µДАлЧУ·ЅіМКЅОЄ_____ЎЈ
ЈЁ3Ј©№¤ТµОІЖшЦРє¬УРґуБїµДµЄСх»ЇОпЈ¬NH3ґЯ»Ї»№ФµЄСх»ЇОпЈЁSCRЈ©јјКхКЗДїЗ°У¦УГЧо№г·єµДСМЖшµЄСх»ЇОпНСіэјјКхЎЈ·ґУ¦ФАнИзНјЛщКѕЈє
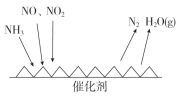
ўЩУЙЙПНјїЙЦЄSCRјјКхЦРµДСх»ЇјБОЄ_____________ЎЈ
ўЪУГFeЧчґЯ»ЇјБјУИИК±Ј¬ФЪ°±ЖшЧгБїµДЗйїцПВЈ¬µ±NO2УлNOµДОпЦКµДБїЦ®±ИОЄ1ЎГ1К±Ј¬РґіцёГ·ґУ¦µД»ЇС§·ЅіМКЅЈє________ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїВИ»ЇВБїЙЦЖ±ёОЮ»ъёЯ·ЦЧУ»мДэјБЈ¬ФЪУР»ъєПіЙЦРУР№г·єµДУГНѕЎЈНкіЙПВБРМоїХЈє
ЈЁ1Ј© КµСйКТЕдЦЖВИ»ЇВБИЬТєК±јУИлСОЛбµДДїµДКЗ____________ЎЈ
ЈЁ2Ј©НщAlCl3ИЬТєЦРјУИл№эБїПВБРИЬТєЈ¬ЧоЦХµГµЅОЮЙ«іОЗеИЬТєµДКЗ______ЈЁМо±аєЕЈ©ЎЈ
a Na2CO3 b NaOH c NaAlO2 d H2SO4
ИЎAlCl3ИЬТєЈ¬УГРЎ»ріЦРшјУИИЦБЛ®ёХєГХфёЙЈ¬ЙъіЙ°ЧЙ«№ММеµДЧйіЙїЙ±нКѕОЄЈєAl2ЈЁOHЈ©nClЈЁ6-nЈ©Ј¬ОЄИ·¶ЁnµДЦµЈ¬ИЎ3.490g°ЧЙ«№ММеЈ¬И«ІїИЬЅвФЪ0.1120 molµДHNO3ЈЁЧгБїЈ©ЦРЈ¬ІўјУЛ®ПЎКНіЙ100 mLЈ¬Ѕ«ИЬТє·ЦіЙБЅµИјЫЈ¬ЅшРРИзПВКµСйЈє
ЈЁ3Ј©Т»·ЭУлЧгБї°±Л®ід·Ц·ґУ¦єу№эВЛЎўПґµУЎўЧЖЙХЈ¬ЧоєуµГAl2O3µДЦКБїОЄ1.020gЎЈЕР¶ПјУИл°±Л®ТСЧгБїµДІЩЧчКЗ________ЎЈ№эВЛЎўПґµУєуЦБЙЩТЄЧЖЙХ_______ґОЈЁМоРґКэЧЦЈ©Ј»Ів¶ЁСщЖ·ЦРВБФЄЛШє¬БїК±І»СЎФсІв¶ЁёЙФпAlЈЁOHЈ©3µДЦКБїЈ¬¶шКЗІв¶ЁA12O3µДЦКБїµДФТтїЙДЬКЗ____ЈЁСЎМо±аєЕЈ©ЎЈ
a ёЙФпAlЈЁOHЈ©3№ММеК±ТЧК§Л® b Al2O3µДЦКБї±ИAlЈЁOHЈ©3ґуЈ¬ОуІоРЎ
c іБµнAlЈЁOHЈ©3К±І»НкИ« d ЧЖЙХСх»ЇВБК±І»·ЦЅв
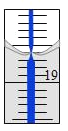
ЈЁ4Ј©ґУБнТ»·ЭИЬТєЦРИЎіц20.00 mLЈ¬УГ0.1290 mol/LµД±кЧјNaOHИЬТєµО¶Ё№эБїµДПхЛбЈ¬µО¶ЁЗ°µО¶Ё№Ь¶БКэОЄ0.00 mLЈ¬ЦХµгК±µО¶Ё№ЬТєГжЈЁѕЦІїЈ©ИзНјЛщКѕЈЁ±іѕ°ОЄ°ЧµЧА¶ПЯµДµО¶Ё№ЬЈ©ЎЈФтµО¶Ё№ЬµД¶БКэ__________mLЈ¬Al2ЈЁOHЈ©nClЈЁ6-nЈ©ЦРnµДЦµОЄ__________ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎї»ЇєПОпWїЙУГЧчёЯ·ЦЧУЕтХНјБЈ¬Т»ЦЦєПіЙВ·ПЯИзПВЈє
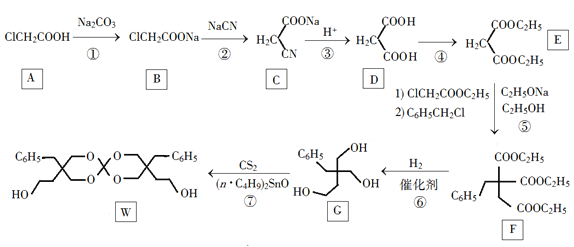
»ШґрПВБРОКМвЈє
ЈЁ1Ј©AµД»ЇС§ГыіЖОЄ________ЎЈ
ЈЁ2Ј©ўЪµД·ґУ¦АаРНКЗ__________ЎЈ
ЈЁ3Ј©·ґУ¦ўЬЛщРиКФјБЈ¬Мхјю·Ц±рОЄ________ЎЈ
ЈЁ4Ј©GµД·ЦЧУКЅОЄ________ЎЈ
ЈЁ5Ј©WЦРє¬Сх№ЩДЬНЕµДГыіЖКЗ____________ЎЈ
ЈЁ6Ј©РґіцУлE»ҐОЄН¬·ЦТм№№МеµДхҐАа»ЇєПОпµДЅб№№јтКЅЈЁєЛґЕ№ІХсЗвЖЧОЄБЅЧй·еЈ¬·еГж»э±ИОЄ1ЎГ1Ј©______________ЎЈ
ЈЁ7Ј©±ЅТТЛбЬРхҐЈЁ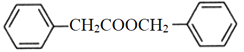 Ј©КЗ»ЁПгРНПгБПЈ¬ЙијЖУЙ±ЅјЧґјОЄЖрКјФБПЦЖ±ё±ЅТТЛбЬРхҐµДєПіЙВ·ПЯ__________ЈЁОЮ»ъКФјБИОСЎЈ©ЎЈ
Ј©КЗ»ЁПгРНПгБПЈ¬ЙијЖУЙ±ЅјЧґјОЄЖрКјФБПЦЖ±ё±ЅТТЛбЬРхҐµДєПіЙВ·ПЯ__________ЈЁОЮ»ъКФјБИОСЎЈ©ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїИЛМеСЄТєµДpHНЁіЈФЪ7.35-7.45Ц®јдµДФТтКЗСЄТєЦРґжФЪNaH2PO4-Na2HPO4µИ»єіеМеПµЎЈіЈОВПВЈєKa1(H3PO4)=7.6ЎБ10-3ЎўKa2(H3PO4)=6.3ЎБ10-8ЎЈПВБРЦё¶ЁИЬТєЦРОўБЈОпЦКµДБїЕЁ¶И№ШПµХэИ·µДКЗ
A.0.1mol/L NaH2PO4ИЬТєЈє2c(HPO42-)+3c(PO43-)Јѕc(Na+)-c(H2PO4-)
B.іЈОВПВЈ¬pH=7µДNaH2PO4єНNa2HPO4µД»мєПИЬТєЈєc(Na+)Јѕc(HPO42-)Јѕc(H2PO4-)
C.Пт10 mL0.1mol/L NaH2PO4ИЬТєЦРјУИл5mL 0.4 mol/L NaOHИЬТєЈєc(H+)+3c(H3PO4)+2c(H2PO4-)+c(HPO42-)=c(OH-)
D.ОпЦКµДБїЕЁ¶ИПаµИNaH2PO4єНNa2HPO4ИЬТєµИМе»э»мєПЈє3[c(H2PO4-)+c(HPO42-)+c(PO43-)]=2c(Na+)
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїH2O2µДЦЖИЎј°ЖдФЪОЫЛ®ґ¦Ан·ЅГжµДУ¦УГКЗµ±З°їЖС§СРѕїµДИИµгЎЈ
(1)Ў°СхТхј«»№Ф·ЁЎ±ЦЖИЎH2O2µДФАнИзМвНјЛщКѕЈє
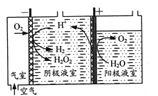
Тхј«±нГж·ўЙъµДµзј«·ґУ¦УРЈє
ўс.2H++O2+2e-=H2O2
ўт. H2O2+2H++ 2e-=2H2O
ўу. 2H+ +2e-=H2Ўь
ўЩРґіцСфј«±нГжµДµзј«·ґУ¦КЅЈє___ЎЈ
ўЪЖдЛыМхјюПаН¬К±Ј¬І»Н¬іхКјpH(ѕщРЎУЪ2)МхјюПВЈ¬H2O2ЕЁ¶ИЛжµзЅвК±јдµД±д»ЇИзНјЛщКѕЈ¬c(H+)№эґу»т№эРЎѕщІ»АыУЪH2O2ЦЖИЎЈ¬ФТтКЗ_______ЎЈ
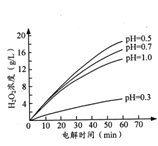
(2)ґжјоРФМхјюПВЈ¬H2O2µДТ»ЦЦґЯ»Ї·ЦЅв»ъАнИзПВЈє
H2O2(aq)+Mn2+(aq)=Ў¤OH(aq)+Mn3+(aq)+OH-(aq) H=akJ/mol
H2O2(aq)+ Mn3+(aq) +2OH-(aq)= Mn2+(aq) +Ў¤O2-(aq) +2H2O(l) H=bkJ/mol
Ў¤OH(aq) +Ў¤O2-(aq)=O2(g) +OH-(aq) H=ckJ/mol
2H2O2(aq)= 2H2O(l)+O2(g) ЎчH=_______ ЎЈёГ·ґУ¦µДґЯ»ЇјБОЄ ____ЎЈ
(3)H2O2ЎўO3ФЪЛ®ЦРїЙРОіЙѕЯУРі¬ЗїСх»ЇДЬБ¦µДфЗ»щЧФУЙ»щ(Ў¤OH)Ј¬їЙУРР§ИҐіэ·ПЛ®ЦРµДґОБЧЛбёщАлЧУ(H2PO2-)ЎЈ
ўЩИхјоРФМхјюПВЎ¤OHЅ«H2PO2-Сх»ЇіЙPO43-Ј¬АнВЫЙПl.7gЎ¤OHїЙґ¦Анє¬0.001mol/L H2PO2-µДДЈДв·ПЛ®µДМе»эОЄ______ЎЈ
ўЪОЄ±ИЅПІ»Н¬Н¶БП·ЅКЅПВє¬H2PO2-ДЈДв·ПЛ®µДґ¦АнР§№ыЈ¬ПтБЅ·ЭµИМе»э·ПЛ®СщЖ·ЦРјУИлµИБїH2O2єНO3Ј¬ЖдЦРТ»·ЭФЩјУИлFeSO4ЎЈ·ґУ¦ПаН¬К±јдЈ¬КµСйЅб№ыИзНјЛщКѕЈє
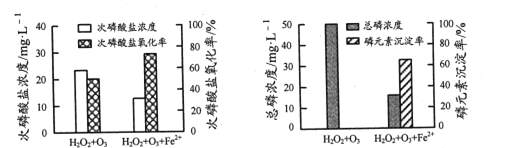
МнјУFeSO4єуЈ¬ґОБЧЛбСОСх»ЇВКЎўБЧФЄЛШіБµнВКѕщПФЦшМбёЯЈ¬ФТтКЗ______ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїДі»ЇєПОп6.4 gФЪСхЖшЦРНкИ«ИјЙХЈ¬Ц»ЙъіЙ8.8 g CO2єН7.2 g H2OЎЈПВБРЛµ·ЁЦРХэИ·µДКЗ(ЎЎЎЎ)
ўЩёГ»ЇєПОпЅцє¬МјЎўЗвБЅЦЦФЄЛШ ўЪёГ»ЇєПОпЦРМјЎўЗвФЧУёцКэ±ИОЄ1ЎГ4
ўЫОЮ·ЁИ·¶ЁёГ»ЇєПОпКЗ·сє¬УРСхФЄЛШ ўЬёГ»ЇєПОпЦРТ»¶Ёє¬УРСхФЄЛШ
A. ўЩўЪB. ўЪўЬC. ўЫўЬD. ўЪўЫ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїНјОЄФЄЛШЦЬЖЪ±нµДТ»Ії·ЦЈ¬ПВБРЛµ·ЁІ»ХэИ·µДКЗ
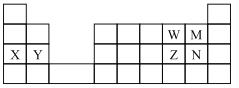
A.ФЧУ°лѕ¶ X>Y
B.XЎўMµДјтµҐАлЧУµДµзЧУІгЅб№№ПаН¬
C.MЧоёЯјЫСх»ЇОпµДЛ®»ЇОпЛбРФ±И N µДЗї
D.WµДјтµҐЗв»ЇОп±ИZµДјтµҐЗв»ЇОпОИ¶Ё
Ійїґґр°ёєНЅвОц>>
№ъјКѧУУЕСЎ - Б·П°ІбБР±н - КФМвБР±н
єю±±КЎ»ҐБЄНшОҐ·ЁєНІ»БјРЕПўѕЩ±ЁЖЅМЁ | НшЙПУРє¦РЕПўѕЩ±ЁЧЁЗш | µзРЕХ©ЖѕЩ±ЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРє¦РЕПўѕЩ±ЁЧЁЗш | ЙжЖуЗЦИЁѕЩ±ЁЧЁЗш
ОҐ·ЁєНІ»БјРЕПўѕЩ±Ёµз»°Јє027-86699610 ѕЩ±ЁУКПдЈє58377363@163.com