
����Ŀ�����ĵ��ʼ����������о����������ж�����;��������ѧ֪ʶ�ش��������⣺
��1��Fe3+�۵����Ų�ʽΪ______________��������Fe2+��ԭ��ǿ��ԭ��_______________��
��2����±������SCN-�Ǽ���Fe3+���Լ�֮һ����SCN-��Ϊ�ȵ������һ�����ʵķ���ʽ Ϊ_____________��
��3������֪Fe3C�ľ����ṹ��̼ԭ�ӵ���λ��Ϊ6����̼ԭ�ӽ��ڵ���ԭ����ɵĿռ乹��Ϊ____________________����ԭ�ӵ���λ����___________��
������ij�ֻ�����ṹ��ʽ��ͼ1��ʾ����������������еĵڶ�����Ԫ��ԭ�ӵĵ縺���ɴ�С��˳��Ϊ_________________________�������������е�ԭ�ӵ��ӻ���ʽΪ_____________����ͼ1���á��������FeԪ����Χ����λ��_____��
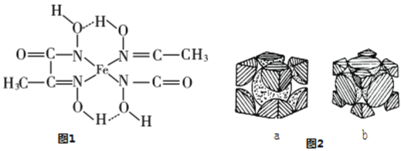
��4�������ʵĶѻ���ʽ���������֣�������ͼ�ֱ���ͼ2a��b��ʾ���������������������ܶѻ�����_______���a����b�������˾�����ԭ�ӵ������ռ��������ı���Ϊ____________________���ú�Բ���ʦеĴ���ʽ��ʾ����
���𰸡�3d5 Fe2+�۵����Ų�Ϊ3d6��ʧȥ1�����ӱ��Fe3+��3d5�ȶ��ṹ�� CS2��CO2��N2O �������� 2 O>N>C sp2��sp3 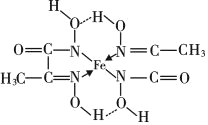 b
b ![]()
��������
���ݺ�������Ų������ȫ�����������ȶ��ṹ������𣻸��ݵȵ�����ԭ��������𣻸��ݾ�����ԭ�Ӹ����ȼ���λԭ�ӵĸ��������𣻸��ݻ�ѧ�������ͼ�����ӻ����۷�����𣻸��ݾ����Ľṹ������������ԭ�ӵĶѻ���ʽ�������
��1��Fe��26��Ԫ�أ�ԭ�ӵĵ����Ų�ʽ��1s22s22p63s23p63d64s2��Feԭ����ʧȥ4s�ܼ�2�����ӡ���ʧȥ3d�ܼ�1�������γ�Fe3+��Fe3+�۵����Ų�ʽΪ��3d5��Fe3+�۵����Ų����ڰ�����ȶ�״̬����Fe2+�۵����Ų�Ϊ3d6��ʧȥ1�����ӱ��Fe3+��3d5�ȶ��ṹ����Fe2+��ԭ��ǿ���ʴ�Ϊ��3d5��Fe2+�۵����Ų�Ϊ3d6��ʧȥ1�����ӱ��Fe3+��3d5�ȶ��ṹ��
��2����SCN��Ϊ�ȵ������һ�����ʣ�������S�滻Nԭ����1����λ����ɣ����ĵȵ�����Ϊ��CS2����һ����Oԭ���滻Sԭ�ӵõ��ȵ�����CO2��2��Nԭ���滻1��Cԭ�ӡ�1��Oԭ�ӵõ��ȵ�����N2O���ȵȣ��ʴ�Ϊ��CS2��CO2��N2O��
��3����Fe3C�ľ����ṹ��̼ԭ�ӵ���λ��Ϊ6����̼ԭ�ӽ��ڵ���ԭ�ӣ���Cԭ��Ϊԭ�㽨����ά����ϵ��Feԭ��λ�����������ҹ���ԭ�㣨̼ԭ�ӣ��Գƣ�6��Fe�γɵĿռ�ṹΪ�������壬��Cԭ�ӵ���λ��Ϊ6����λ��֮�ȵ�����Ӧԭ����Ŀ���ȣ���Feԭ����λ��Ϊ6��1��3=2���ʴ�Ϊ���������壻2��
��ͬ��������Ԫ�ش������ҵ縺�������ʵ縺�Դ�СΪO>N>C�����������γ�C��N˫����Nԭ���γ�2������������1�Թµ��Ӷԣ����γɵ�����Nԭ�ӣ��γ�2������������2�Թµ��Ӷԣ��ӻ������Ŀ�ֱ�Ϊ3��4��Nԭ�Ӳ�ȡsp2��sp3�ӻ����γ�8�����ȶ��ṹʱ��Nԭ���γ�3�����ۼ�������Nԭ���γ�4����������1����Nԭ�������������γɵ���λ���������λ��Ϊ�� ���ʴ�Ϊ��O>N>C��sp2��sp3��
���ʴ�Ϊ��O>N>C��sp2��sp3�� ��
��
��4�������������ܶѻ���ԭ�Ӵ��ھ����Ķ��������ģ�ͼa��Feԭ�Ӵ��ڶ��������ģ������������ѻ���ͼb��Feԭ�Ӵ��ڶ��������ģ����������������ܶѻ��������������ܶѻ��д��ھ�����Խ�����Feԭ�ӽ������ڣ���Feԭ�Ӱ뾶Ϊr�����ⳤ��4r��![]() =
=![]() �����������
�����������![]() ��������Feԭ����Ŀ��8��
��������Feԭ����Ŀ��8��![]() +6��
+6��![]() =4��������Feԭ���������4��
=4��������Feԭ���������4��![]() 3��r3��������ԭ�ӵ������ռ��������ı���Ϊ��
3��r3��������ԭ�ӵ������ռ��������ı���Ϊ��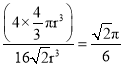 ���ʴ�Ϊ��b��
���ʴ�Ϊ��b��![]() ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ĥ����ԭ���͵绯ѧԭ���Ʊ������������ɫ������N2O5��װ����ͼ��ʾ������˵������ȷ����

A. �缫a�͵缫c������������Ӧ
B. �缫d�ĵ缫��ӦʽΪ2H++2e-=H2��
C. c�缫�ϵĵ缫��ӦʽΪN2O4-2e-+H2O=N2O5+2H+
D. װ��A��ÿ����64gSO2��������װ��A��װ��B�о���2moH+ͨ�����ӽ���Ĥ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���0.10 mol��L��1KOH��Һ�ζ�10.00 mL 0.10 mol��L��1H2C2O4(��Ԫ����)��Һ���õζ�������ͼ(�����Һ������ɿ��ɻ��ǰ��Һ�����֮��)����ش��������⣺
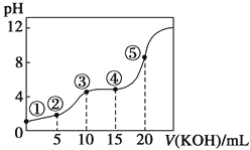
��1�������ʾ��Һ�У�Kw��__________��
��2�������ʾ��Һ�еĵ���غ�ʽΪ______________________________________��
��3�������ʾ��Һ�д���________��ƽ�⡣
��4�������ʾ��Һ�е������غ�ʽΪ0.10 mol��L��1��___________________________��
��5�������ʾ��Һ�и�����Ũ�ȵĴ�С˳��Ϊ________________________________��
��6������5����ʾ��Һ�У�ˮ�ĵ���̶�������_______����С����________(����Żش�)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1�������£�0.1mol/L ��CH3COOH ��Һ����1% CH3COOH ���ӷ������룬����Һ��pH=______________������ʹ0.10mol��L-1 CH3COOH�ĵ���̶��������____________����
a.��������0.10 mol��L-1��ϡ���� b.����CH3COOH��Һ
c.��ˮϡ����0.010 mol��L-1. d.��������������
e.���������Ȼ��ƹ��� f.��������0.10 mol��L-1��NaOH��Һ
��2������������пͶ��������pH ������3 �Ĵ����������Һ�У�������ַ�Ӧ����ֻ��һ����Һ��п��ʣ�࣬�����������������V( ����)________V(����)(����>������ <�� ����=��)��
��3��0.1mol/L ��ij��H2A ��pH=4����H2A �ĵ��뷽��ʽΪ_________________��
��4��ij�¶��£�Kw=1��10-12����0.02mol/L��Ba(OH)2��Һ������ʵ���Ũ�ȵ�NaHSO4��Һ�������ϣ����û��Һ��pH=____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ�����ӵ�������ֵ�����������������

A����16 g��ԭ�ӵĶ������辧���к��еĦļ���ĿΪ2NA
B��23.4 g NaCl�����к���0.1NA����ͼ��ʾ�Ľṹ��Ԫ
C�����³�ѹ�£�5 g D2O���е�������������������������Ϊ2.5NA
D��2 mol SO2��1 mol O2��һ�������·�Ӧ���û�����������С��2NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ��ˮ�г�����һ������Cr2O72-��CrO42-�����ǻ�����༰��̬ϵͳ�����ܴ�Σ����������д��������õĴ������������֣�����1����ԭ��������
�÷��Ĺ�������Ϊ��
���еڢٲ�����ƽ��2CrO42����ɫ��+2H+Cr2O32����ɫ��+H2O
��1����ƽ����ϵ��pH=2������Һ��______ɫ��
��2����˵���ڢٲ���Ӧ��ƽ��״̬����_____������ţ�
A��Cr2O72��CrO42��Ũ����ͬ
B��2v��Cr2O72��=v��CrO42��
C����Һ����ɫ����
��3���ڢڲ��У���ԭ1molCr2O72���ӣ���Ҫ______mol��FeSO47H2O��
��4���ڢ۲����ɵ�Cr��OH��3����Һ�д������³����ܽ�ƽ�⣺Cr��OH��3��s��Cr3+��aq��+3OH-��aq���������£�Cr��OH��3���ܶȻ�Ksp=c��Cr3+��c3��OH-��=10-32��Ҫʹc��Cr3+������10-5mol/L����Һ��pHӦ����______��
����2����ⷨ��
�÷���Fe���缫��⺬Cr2O72�����Է�ˮ�����ŵ��Ľ��У�������������ҺpH���ߣ�����
Cr��OH��3������
��5����Fe���缫��ԭ��Ϊ______���õ缫��Ӧʽ���ͣ���
��6��������������ҺpH���ߣ���Һ��ͬʱ���ɵij�������______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л�������F��һ����Ҫ���л��ϳ��м��壬��ϳ�·������ͼ��ʾ��
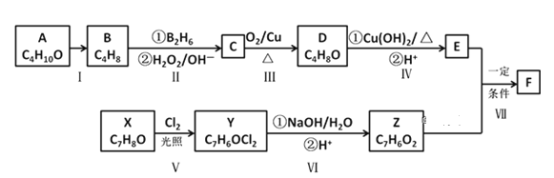
��֪����A�ĺ˴Ź�������ͼ����ʾ�����
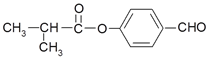
��F�Ľṹ��ʽΪ��
��ͨ����ͬһ��̼ԭ�������������ǻ����ȶ�������ˮ�γ��ʻ���
��R-CH=CH2![]() R-CH2CH2OH
R-CH2CH2OH
��ش��������⣺
(1)A������Ϊ______________(ϵͳ������)��Z�����������ŵ�������___________��
(2)��Ӧ���ķ�Ӧ������__________��
(3)E�Ľṹ��ʽΪ_______________________��
(4)д����Ӧ���Ļ�ѧ����ʽ____________________________________________��
(5)д����ӦIV���Ļ�ѧ����ʽ____________________________________________��
(6)W��Z��ͬϵ���Է���������Z��14����W��ͬ���칹������������������
���ܷ���������Ӧ���ڱ�����������ȡ�������۲���ˮ�⣬��FeCl3��Һ����ɫ�Ľṹ����_________��(�����������칹)���˴Ź��������������ĽṹΪ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�������ʵ����˵��NH3��H2OΪ������ʵ���
A. 0.1 mol��L-1NH3��H2O��pH��13
B. 0.1 mol��L-1 NH4Cl��Һ��pHС��7
C. ��ͬ�����£�Ũ�Ⱦ�Ϊ0.1 mol��L-1 NaOH��Һ�Ͱ�ˮ����ˮ�ĵ���������
D. 0.1 mol��L-1NH3��H2O��ʹ��ɫ��̪��Һ���ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������H+����Cl������Al3������K������S2������OH������NO3������NH4���ֱ����H2O�У������ϲ�Ӱ��ˮ�ĵ���ƽ��������ǣ� ��
A.�٢ۢݢߢ�B.�ڢܢޢ�C.�٢�D.�ڢܢ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com