
��18�֣���ҵ�ϴӵ�⾫��ͭ�������ࣨ��������ͭ�����ȵ��ʣ�����ȡ����ʪ�������������£�
��1������ҺX�м�����м��������______ ���˲����в��ܼ���������۵�ԭ����______��
��2��������ҺZ�������ӵIJ���������______��
��3�����˲�����Ҫ�õ��������������������õ���������ʵ��������______��
��4��ʵ��������ȡSO2��ԭ��Ϊ�� ���˴�Ӧʹ��______�����Ũ�����ᡱ��ϡ���ᡱ����ԭ���� ______����ȡSO2��װ�ã����ѡ����ͼ�е�______��
���˴�Ӧʹ��______�����Ũ�����ᡱ��ϡ���ᡱ����ԭ���� ______����ȡSO2��װ�ã����ѡ����ͼ�е�______��
��5�����������ĺ����������·����ⶨ��
 ͨ����Na2S2O3����Һ���Լ��ԣ��ζ���Ӧ�������ɵ�I2���������ĺ������ζ��������õ��IJ���������_______��ʵ����ȷ����0.1200g������Ʒ���ζ�������0.2000mol
ͨ����Na2S2O3����Һ���Լ��ԣ��ζ���Ӧ�������ɵ�I2���������ĺ������ζ��������õ��IJ���������_______��ʵ����ȷ����0.1200g������Ʒ���ζ�������0.2000mol ��Na2S2O3��Һ27.60mL���������Ʒ��������������Ϊ ��
��Na2S2O3��Һ27.60mL���������Ʒ��������������Ϊ ��
��18�֣�
��1����SeO42-��ԭΪSeO32-��2�֣� �������ۻὫCu2+��SeO32-����ԭΪ���ʣ����������ķ��루2�֣�
��2��ȡ��ҺZ���������Թ��У��μ������ữ��BaCl2��Һ���а�ɫ�������ɣ�˵����Һ�к���SO42-��2�֣�
��3�������ᴿ��һ�����ʵ���Ũ����Һ�����ơ����ʵ��ܽ⡢��Һ��������Ũ�����ϡ�͵ȣ�2�֣�ֻҪ��ȷ���������֣�
��4����Ũ�����ᣨ2�֣� SO2������ˮ���ý�Ũ������������ SO2���ݳ���2�֣�
b��2�֣�
��5����ƿ����ʽ�ζ��ܣ�2�֣� 90.85%��2�֣�
���������������1��H2O2Ϊǿ���������ɽ��������е�Se��������ΪSeO42-����������ͼ������������м������SeO32-��������м�������ǣ���SeO42-��ԭΪSeO32-��������������������Cu2+��SeO32-��Ӧ��������ԭΪ���ʣ����������ķ��롣
��2����ҺZ���е�������ΪSO42?�����鷽��Ϊ��ȡ��ҺZ���������Թ��У��μ������ữ��BaCl2��Һ���а�ɫ�������ɣ�˵����Һ�к���SO42-��
��3�������ᴿ����Һ���������ò��������裬ʹ��Һ�������ȣ�һ�����ʵ���Ũ����Һ�����ƣ��ò��������衢���������ʵ��ܽ⡢Ũ�����ϡ�͵ȡ�
��4����ΪSO2������ˮ���ý�Ũ������������ SO2���ݳ���������ȡSO2�ý�Ũ�����a������������ڿ�״������Ƚϴ�Ĺ�����Һ�巴Ӧ��Na2SO3Ϊ��ĩ״�����ʺϣ�b����Һ©���ʺϷ�ĩ״������Һ�巴Ӧ��Һ©���ɿ���Һ��ļ�������c������©���ĵ��ܿ���Һ�����ϣ�����ӳ���©���ݳ������ʺϣ�d������©�����ܿ���Һ��ļ���������������ʵ�Ϊb�
��5��Na2S2O3����ҺΪ���ԣ��ü��Եζ���ʢ�ţ�����Һ����ƿʢ�ţ�������Ŀ����3����ѧ����ʽ�ɵö�Ӧ��ϵ��Se ~ SeO2 ~ 2I2 ~ 4Na2S2O3���������Ʒ��������������=0.0276L��0.2000mol/L��1/4��79g/mol��0.1200g��100%=90.85%
���㣺���⿼��ʵ���������������������ʵ�鷽����������������ѧ���㡣


 Ӣ�żƻ���ĩ����ϵ�д�
Ӣ�żƻ���ĩ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�������ʵ����淽������ȷ����( )
| A����������Һ�ͼ�����Һ����ڽ����ܷ���Լ�ƿ�� |
| B�������ʢ������ɫ�Լ�ƿ�� |
| C��Һ��ʢ���ڴ��н�����ϸ��ƿ�� |
| D����ˮʢ������ɫϸ��ƿ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ѧʵ��С��̽������ʳ�ð״��д���ĵ�ȷŨ�ȣ�ȡ25.00mLijƷ��ʳ�ð״�����ƿ�У���ʵ������Ũ��Ϊc��mol/L�ı�NaOH��Һ������еζ���
��1����ͼ��ʾ50mL�ζ�����Һ���λ�ã���A��C�̶ȼ����l mL��A���Ŀ̶�Ϊ25���ζ�����Һ�����ӦΪ����������mL��
��2��Ϊ�˼�Сʵ������ͬѧһ������������ʵ�飬����ÿ����ȡ�״������ΪVmL��NaOH��ҺŨ��Ϊc mo1/L������ʵ������¼���£�
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����NaOH��Һ���/mL | 26.02 | 25.35 | 25.30 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������п�к�����CuO��Fe3O4��SiO2�����ʡ���ҵ���Դ�����п��������п���壨ZnSO4��7H2O���Ĺ�����������ͼ��ʾ�� ��
��
��֪�������£���Һ�е�Fe3+��Zn2+��Fe2+������������ʽ��ȫ������pH�ֱ�Ϊ��3.7��6.5��9.7��
��ش��������⣺
��1�������۵������� ��
��2����������п�۵�����Ϊ ��
��3������30%H2O2��Ӧ�����ӷ���ʽ�� ��
��4����������Ca(OH)2������ҺpH���ٽ�Fe3+ˮ�⣬Fe3+ˮ�ⷴӦ��ƽ�ⳣ������ʽK���� ����Ca(OH)2���ܹ�����ԭ������ ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��8�֣���NaCl��������1mol/L��NaCl��Һ100mL��
��1��ʵ�����������������ٲ��������ڽ�ͷ�ιܣ���100mL��Ͳ����100mL����ƿ����250mL��Ͳ����������ƽ����50mL�ձ���Ӧѡ�õ����������ţ� ��
��2��Ӧ��ȡNaCl������Ϊ ��
(3)�ڶ���ʱ����С�ĵμӵ�����ˮ�����˿̶��ߣ������ķ����� ����NaCl��Һת�Ƶ�����ƿ��δ���ձ��Ͳ���������ϴ�ӣ���������Һ��Ũ��(�ƫ�ߡ���ƫ�͡����䡱) ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
(19��)��������(��Ҫ�ɷ�ΪFeS2)Ϊԭ���Ʊ��Ȼ�������(FeCl3��6H2O)�Ĺ����������£�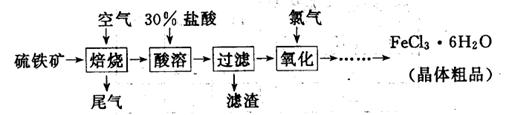
(1)���������з�����Ӧ�����ӷ���ʽ�� �������������ɵ������ӵ��Լ��� ��
(2)β������Ҫ��N2��O2��SO2��������CO2��H2O��ȡ��״���µ�β��V L�ⶨSO2������
����һ����β������ͨ������װ�á�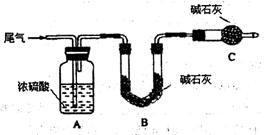
��C������������ ����װ�õ������� ��
��ʵ��ʱ��ͨ��β������ͨ��һ������������ͨ��Bװ�õ�����������SO2���������������Ϊ�÷����Ƿ���� ����˵������ (�����������ÿղ�����д)��
����������β������ͨ��������ˮ�������õ���Һ�м�������Ȼ�����Һ���ˣ�������ϴ�ӡ�����Ƶ�������Ϊm g��
�ټ�������Ȼ�����Һ��Ŀ���� ��
�ڽ��г���ϴ�ӵķ����� ��
��SO2�����ı���ʽ�� ���ú�m��V�Ĵ���ʽ��ʾ)��
(3)��FeCl3��Һ�еõ�FeCl3 6H2O����IJ������� ����ȴ�ᾧ�����ˣ��ù����豣�������������ϱ�Ҫ�����ӷ���ʽ˵��ԭ�� .
6H2O����IJ������� ����ȴ�ᾧ�����ˣ��ù����豣�������������ϱ�Ҫ�����ӷ���ʽ˵��ԭ�� .
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����KMnO4��Һ������ᣨH2C2O4����Һ��Ӧ��ij̽��С�����÷�Ӧ��������Һ��ɫ��ʧ�����ķ������о�Ӱ�췴Ӧ���ʵ����ء�
��.ʵ��ǰ������Ũ��Ϊ0.1000mol?L-1����KMnO4����Һ�ζ�δ֪Ũ�ȵIJ��ᡣ
��1��д���ζ������з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2���ζ������в����ζ��ܵ�ͼʾ��ȷ���� ��
��3�����ζ�ǰ�ζ��ܼ��촦�����ݣ��ζ�����ʧ����ʹ��õIJ�����ҺŨ��
���ƫ�ߡ�����ƫ�͡������䡱����
��.ͨ���ζ�ʵ��õ�������Һ��Ũ��Ϊ0.2000mol��L-1 ���øò�����Һ���±����к���ʵ�飨ÿ��ʵ�������Һ��������Ϊ8mL����
| ʵ���� | �¶ȣ��棩 | ���� ����(g) | ���Ը��������Һ | ʵ��Ŀ�� a. ʵ��1��2̽�� �� b. ʵ��1��3̽����Ӧ��Ũ�ȶԸ÷�Ӧ���ʵ�Ӱ�죻 c. ʵ��1��4̽�������Ը÷�Ӧ���ʵ�Ӱ�졣 | |
| ��� ��mL�� | Ũ�� (mol?L-1) | ||||
| 1 | 25 | 0.5 | 4 | 0.1000 | |
| 2 | 50 | 0.5 | 4 | 0.1000 | |
| 3 | 25 | 0.5 | 4 | 0.0100 | |
| 4 | 25 | 0 | 4 | 0.1000 | |

| ʵ���� | ��Һ��ɫ����ʱ�䣨min�� | ||
| ��1�� | ��2�� | ��3�� | |
| 1 | 14.0 | 13.0 | 11.0 |
| 3 | 6.5 | 6.7 | 6.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ѧ��һ����ʵ��Ϊ������ѧ�ƣ���ѧ��ȡ�õķ�˶�ɹ�������ʵ�����Ҫ���÷ֲ����ġ��������ʵ��װ��ͼ�ش����⣺ 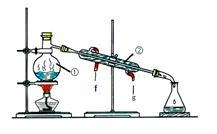
I II
��1��д������ͼ�����������ƣ��� ���� ��
��2��������װ��I�������ᣨ�е�118�棩�������������е�77.1�棩�Ļ�����ȱ�ٵ������� ��������������������е�ʵ�����������Ϊ ��ʵ��ʱ����������ȴˮ�Ľ���Ϊ (ѡ� f �� �� ��g �� )��
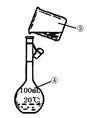
��3����������250 mL 0.2 mol��L��1 NaCl��Һ��װ��II��ijͬѧת����Һ��ʾ��ͼ��ͼ������������ֱ��� �� ��
��4�����в�����ʹ������ҺŨ��ƫС���� (����ţ���
���ձ���NaOH��Һ��������ƿ��û��ϴ���ձ�
�ڶ��ݺ�ҡ�ȣ�����Һ����ڿ̶��ߣ��ٵμ�����ˮ���̶���
��ʵ���õ�����ƿϴ����δ������溬������ˮ
�ܶ���ʱ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���к�CaO���ʵ�CaC2������ij�о���ѧϰС���ͬѧ�����������ַ����ⶨCaC2�����Ĵ��ȡ�����д���пհף�
��1����һ�ַ����������ͼ��ѡ���ʵ���װ�ã����һ��ʵ�飬�ⶨCaC2�����Ĵ��ȡ�
��ѡ��װ�õ�����˳��Ϊ������ӿڵ���ĸ���� ��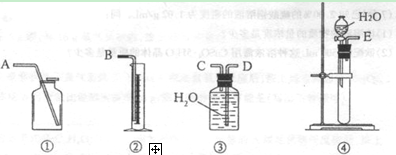
��2���ڶ��ַ���������������ˮ����ƿ�з�Ӧǰ�������ı仯���ⶨCaC2�������������ȳ�ȡ����1.50g����������ƿ��ˮ������Ϊ195.00g���ٽ�����������ƿ�У���Ӧ������ÿ����ͬʱ���õ��������±���
| | �������� | ����/g |
| ��ƿ��ˮ������ | ��1�� | 196.30 |
| ��2�� | 196.15 | |
| ��3�� | 196.05 | |
| ��4�� | 196.00 | |
| ��5�� | 196.00 |

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com