
�����ȼ���(��ͼ)��100 mL 0.50 mol��L��1��CH3COOH��Һ��100 mL 0.55 mol��L��1��NaOH��Һ��ϣ��¶ȴ�298.0 K������300.7 K����֪���ȼƵ����ݳ���(���ȼƸ�����ÿ����1 K����Ҫ����)��150.5 J��K��1����Һ�ܶȾ�Ϊ1 g��mL��1��������Һ�ı�����c��4.184 J��(g��K)��1��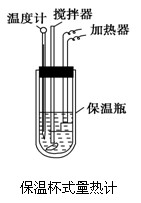
(1).����CH3COOH���к��Ȧ�H������ֵʽ��
(2).������ֵ�����57.3 kJ/mol��ƫ�����ԭ�������
a��ʵ��װ�ñ��¡�����Ч����
b������0.55 mol/L NaOH��Һʱ���ӿ̶��߶���
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
e������Ͳ��ȡNaOH��Һ�����ʱ���Ӷ���
 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��������ѧ��һ��㡢ʵ�������ĩ��ѧ�Ծ����������� ���ͣ�������
��֪1mol SO2(g)����Ϊ1mol SO3�Ħ�H=-99kJ��mol-1.��ش��������⣺
��1����֪�������ȼ����Ϊ296 KJ��mol-1��������S(s)����3 molSO3(g)�ġ�H =
�����ȼ���(��ͼ)��100 mL 0.50 mol/L��CH3COOH��Һ��100 mL 0.55 mol/L NaOH��Һ��ϣ��¶ȴ�298.0 K���ߵ�300.7 K����֪���ȼƵ����ݳ���(���ȼƸ�����ÿ����1 K����Ҫ������)��150.5 J/K����Һ�ܶȾ�Ϊ1 g/mL��������Һ�ı�����c��4.184 J/(g��K)��
(2) CH3COOH���к��Ȧ�H��_______________________________.
��3��CH3COOH���к��ȵ�����ֵΪ��56.1 kJ/mol������Ϊ(1)�в�õ�ʵ��ֵƫ����ܵ�ԭ���ǣ�����㣩____________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�찲��ʡ��һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
��֪1mol SO2(g)����Ϊ1mol SO3�Ħ�H=-99kJ��mol-1.��ش��������⣺
��1����֪�������ȼ����Ϊ296 KJ��mol-1��������S(s)����3 molSO3(g)�ġ�H =
�����ȼ���(��ͼ)��100 mL 0.50 mol/L��CH3COOH��Һ��100 mL 0.55 mol/L NaOH��Һ��ϣ��¶ȴ�298.0 K���ߵ�300.7 K����֪���ȼƵ����ݳ���(���ȼƸ�����ÿ����1 K����Ҫ������)��150.5 J/K����Һ�ܶȾ�Ϊ1 g/mL��������Һ�ı�����c��4.184 J/(g��K)��
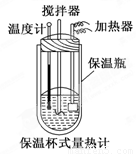
(2) CH3COOH���к��Ȧ�H��_______________________________.
��3��CH3COOH���к��ȵ�����ֵΪ��56.1 kJ/mol������Ϊ(1)�в�õ�ʵ��ֵƫ����ܵ�ԭ���ǣ�����㣩____________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�콭��������ѧ��һ��㡢ʵ�������ĩ��ѧ�Ծ��������棩 ���ͣ�������
��֪1mol SO2(g)����Ϊ1mol SO3�Ħ�H=-99kJ��mol-1.��ش��������⣺
��1����֪�������ȼ����Ϊ296 KJ��mol-1��������S(s)����3 molSO3(g)�ġ�H =
�����ȼ���(��ͼ)��100 mL 0.50 mol/L��CH3COOH��Һ��100 mL 0.55 mol/L NaOH��Һ��ϣ��¶ȴ�298.0 K���ߵ�300.7 K����֪���ȼƵ����ݳ���(���ȼƸ�����ÿ����1 K����Ҫ������)��150.5 J/K����Һ�ܶȾ�Ϊ1 g/mL��������Һ�ı�����c��4.184 J/(g��K)��
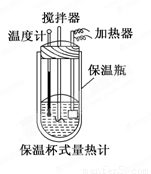
(2) CH3COOH���к��Ȧ�H��_______________________________.
��3��CH3COOH���к��ȵ�����ֵΪ��56.1 kJ/mol������Ϊ(1)�в�õ�ʵ��ֵƫ����ܵ�ԭ���ǣ�����㣩____________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ר���� ���ͣ�ʵ����
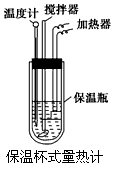
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com