
���� �����������֬���߲˸���ά���غ���Ԫ�أ����������ࣻ
�ڵ�̼������ָ������ϢʱҪ�������������ĵ��������ر��Ƕ�����̼���ŷ������Ӷ���̼�����ٶԴ�������Ⱦ��������̬��
��ΪLED���ṩ���װ�ý������������ɽ�̫����ת��Ϊ����ȥ������
��� �⣺���������ֲ���ͣ�������֬���߲˸���ά���غ���Ԫ�أ����������ۣ������������࣬
�ʴ�Ϊ��C��A��
��A����Լʹ��ֽ�ţ����Լ���ɭ�ֵĿ�������ľ����ͨ������������ն�����̼��ѭ��ʹ�û����ֲ������ģ����ϡ���̼��������A��ȷ��
B��������մ����������ܱ��Ϊ��������Դ�ij�����ü�������������Ҫ���壬���ϡ���̼��������B��ȷ��
C������ʹ�����ϴ����ܼ��ٰ�ɫ��Ⱦ�����ϡ���̼��������C��ȷ��
D������ʹ��һ����ֽ����������ɭ�ֵĿ��������ĵ�������������̼���ŷţ����ϡ���̼��������D��ȷ��
�ʴ�Ϊ��ABCD��
���Ϻ�������ʹ�õĺܶ�LED�ƣ�Ϊ���ṩ���װ�ý������������ɽ�̫����ת��Ϊ���ܣ�
�ʴ�Ϊ��̫���ܣ�
���� ���⿼�黯ѧ������������ϵ���йػ���֪ʶ���ѶȲ����������ǵ�̼��������õ�̼��������ָ���Լ��Ļ���ѽ��ܼ��ŵĴ�ʩ���õ�������������ȥ��


 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| Ӧ��ȡŨ�������/mL | Ӧѡ������ƿ�Ĺ��/mL | ������ƿ���ձ����Ҫ�������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ԣ�HI��HBr��HCl��HF | B�� | ���ԣ�Be��OH��2��Mg��OH��2��KOH | ||
| C�� | �����ԣ�S��O2��F2 | D�� | ��ԭ�ԣ�O2-��S2-��I- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ����
���� ����
�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
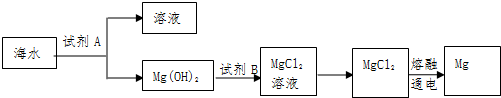
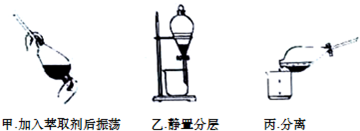
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | 0.1mol/L | B�� | 0.2mol/L | C�� | 0.15mol/L | D�� | 0.05mol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����Ӧ��������������������������ӦΪ���ȷ�Ӧ | |
| B�� | ��ع���ʱ����ѧ��ת��ɵ��� | |
| C�� | ��ɫֲ�������ù�����̫����ת��ɻ�ѧ�� | |
| D�� | ��������ֻ��ͨ�������������ܵ����ʵ�ֱ��ȼ��ת�������ܴӶ������� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com