
����β����NOx�����������������������㷺��ע��
��1��ij��ȤС����������������Ϣ��
N2(g)+O2(g)=2NO(g) ��H=+180.5kJ/mol
2H2(g)+O2(g)=2H2O(g)) ��H=�D483.6kJ/mol
��Ӧ2H2(g)+2NO(g)=2H2O(g)+N2(g) ��H= ��
��2����С�����õ��ԭ���������ͼ1װ�ý���H2��ԭNO��ʵ��[�����ӵ����Ե�SCY�մ�(�ܴ���H+)Ϊ���ʣ������ٱ�Ĥ���缫]��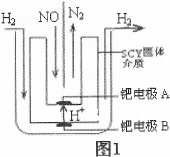
�ٵ缫AΪ �����缫��ӦʽΪ ��
��3����������ԭNOԭ�����£�
����Ӧ��4NO(g)+4NH3(g)+O2(g) 4N2(g)+6H2O(g) (��H <0)
4N2(g)+6H2O(g) (��H <0)
����Ӧ��4NH3(g)+3O2(g) 2N2(g)+6H2O(g)
2N2(g)+6H2O(g)
4NH3(g)+ 4O2(g) 2N2O(g)+6H2O(g)
2N2O(g)+6H2O(g)
4NO(g)+4NH3(g)+3O2(g) 4N2O(g)+6H2O(g)
4N2O(g)+6H2O(g)
�й�ʵ�������ͼ2��ͼ3��ʾ���ݴ˻ش��������⣺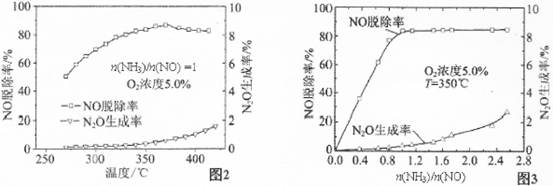
�ٴ���ԭNOӦ����n(NH3)/n(NO)�����ֵΪ �������� ��
������Ӧƽ�ⳣ������ʽ��K= �������¶ȵ����ӣ�K�� (ѡ����ӡ��� ����С�����䡱��
��Ӱ��N2O�����ʵ������� ������Ũ�Ⱥ� ��
��1���D664.1kJ/mol (2��)
��2������(2��) 2NO+4H++4e��=N2��+2H2O (2��)
��3����1 (1��) n(NH3)/n(NO)С��1ʱ��NO�ѳ��ʲ��ߣ�n(NH3)/n(NO)����1ʱ��NO�ѳ������Ӳ�������N2O�������������ӣ� 3�֣�
�� (2��) ��С(2��)
(2��) ��С(2��)
���¶�(1��) n(NH3)/n(NO) (1��)(�������մ�˳��ɵߵ�)
���������������1�����ݸ�˹���ɣ�����2����Ӧ��ȥ��1����Ӧ�ɵã���H=�D664.1kJ/mol����2����ԭ���ԭ����֪���ڵ�·��������������������ͼ��֪���ٵ缫AΪ�������ٵ缫BΪ�������ɵ��ӡ���ɡ�ԭ�Ӿ��غ��֪�����Ի��������������Ļ�ԭ��ӦʽΪ2NO+4H++4e��=N2��+2H2O����3���ٶ�ͼ���Աȿ�֪��n(NH3)/n(NO)С��1ʱ��NO�ѳ��ʲ��ߣ�n(NH3)/n(NO)����1ʱ��NO�ѳ������Ӳ�������N2O�������������ӣ����n(NH3)/n(NO)�����ֵΪ1�����ɷ�Ӧʽ��֪��K= �����ڡ�H<0������Ӧ���ȣ�����ƽ�����ƣ�K��С�����������Ϣ��֪��Ӱ��N2O�����ʵ��������¶ȡ�n(NH3)/n(NO) ������Ũ�ȡ�
�����ڡ�H<0������Ӧ���ȣ�����ƽ�����ƣ�K��С�����������Ϣ��֪��Ӱ��N2O�����ʵ��������¶ȡ�n(NH3)/n(NO) ������Ũ�ȡ�
���㣺�����˹���ɡ�ԭ��ء���ѧƽ������֪ʶ��


 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
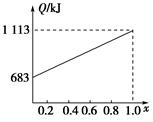
ij������ȼ���ɼס��������л����϶��ɣ��ס����������ʺ���C��H��O����Ԫ���е����ֻ����֡���֪�ס��Ҽ�CO��H2��ȼ�������£�
| ���� | �� | �� | CO | H2 |
| ȼ����/(kJ��mol��1) | 1 366 | 5 518 | 283 | 286 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�о���ѧ��Ӧ�е������仯����Ҫ���塣�����ѧ��֪ʶ�ش��������⣺
��1����֪һ����̼��ˮ������Ӧ���̵������仯����ͼ��ʾ��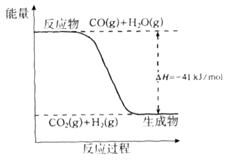
�ٷ�Ӧ���Ȼ�ѧ����ʽΪ____________________________________________��
����֪��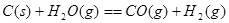
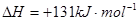
��
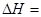
��2����ѧ��Ӧ����Ϊ�ɼ����Ѻ��¼��γɵĹ��̡���ѧ���ļ������γɣ����1 mol��ѧ��ʱ�ͷţ������գ�����������֪��N��N���ļ�����948.9kJ��mol��1��H��H���ļ�����436.0 kJ��mol��1�� N��H���ļ�����391.55 kJ��mol��1����1/2N2(g) + 3/2H2(g) ="=" NH3(g) ��H = ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ҫ��д�����з���
��1��̼������Һ�ʼ��Ե�ԭ�������ӷ��̱�ʾ ��
��2����п������������ʩ�����и��������ĵ缫��ӦΪ�� ��
��3����20.0 g NaOH��ϡ��Һ��ϡ������ȫ�кͣ��ų�28.7 kJ�����������ʾ�÷�Ӧ�к��ȵ��Ȼ�ѧ����ʽ ��
��4��������þ�ܽ���Ũ���Ȼ����Һ�������ӷ��̱�ʾ ��
��5�� Al��OH��3�ĵ��뷴Ӧ����ʽ: ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����֪��Ӧ�����������л�ѧ������ʱ�������仯������ʾ��
H2(g)��Cl2(g)��2HCl(g) ��H����184kJ/mol
4HCl (g)��O2(g)��2Cl2 (g) ��2H2O (g) ��H����115.6kJ/mol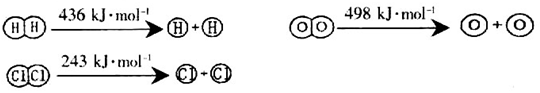
��H2��O2��Ӧ������̬ˮ���Ȼ�ѧ����ʽΪ_________________________________��
�ڶϿ�1mol H��O����������ԼΪ_________________________kJ��
��2����֪ij��Ӧ��ƽ�ⳣ������ʽΪ��K�� ,������Ӧ�Ļ�ѧ����ʽΪ________________��
,������Ӧ�Ļ�ѧ����ʽΪ________________��
��3����֪��ӦN2(g)��3H2(g) 2NH3(g) ��H��0��400��ʱK��0.5������������0.5L���ܱ������н��и÷�Ӧ��һ��ʱ����N2��H2��NH3�����ʵ����ֱ�Ϊ2mol��1mol��2mol�����ʱ��Ӧ��(N2)��______��(N2)�棨�����������������������ʹ�ø÷�Ӧ�Ļ�ѧ��Ӧ���ʼӿ죬ͬʱʹƽ��ʱNH3������ٷ������ӣ��ɲ�ȡ�Ĵ�ʩ��_______������ţ���
2NH3(g) ��H��0��400��ʱK��0.5������������0.5L���ܱ������н��и÷�Ӧ��һ��ʱ����N2��H2��NH3�����ʵ����ֱ�Ϊ2mol��1mol��2mol�����ʱ��Ӧ��(N2)��______��(N2)�棨�����������������������ʹ�ø÷�Ӧ�Ļ�ѧ��Ӧ���ʼӿ죬ͬʱʹƽ��ʱNH3������ٷ������ӣ��ɲ�ȡ�Ĵ�ʩ��_______������ţ���
A.��С�������ѹǿ B.�����¶�
C.�Ӵ��� D.ʹ����Һ������
��4����һ��������ܱ������н������»�ѧ��Ӧ��A(g)��3B(g) 2C(g)��D(s) ��H���仯ѧƽ�ⳣ��K��T�Ĺ�ϵ���±���
2C(g)��D(s) ��H���仯ѧƽ�ⳣ��K��T�Ĺ�ϵ���±���
| T/K | 300 | 400 | 500 | ���� |
| K/(mol��L��1)2 | 4��106 | 8��107 | 1.2��109 | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����̲��Ŵ����ġ���ȼ�������ü�����ˮú����CO��H2�����ٺϳɼ״����������湩Ӧ���ŵ�ȼ�͡�
��֪���� CH4(g)��H2O (g)��CO (g)��3H2 (g) ��H1��+206.2kJ��mol-1
�� CH4(g)��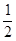 O2(g)��CO(g)��2H2(g) ��H2=��35.4 kJ��mol-1
O2(g)��CO(g)��2H2(g) ��H2=��35.4 kJ��mol-1
�� CH4 (g)��2H2O (g)��CO2 (g)��4H2 (g) ��H3��+165.0 kJ��mol-1
��1��CH4(g)��CO2 (g)��Ӧ����CO(g)��H2(g)���Ȼ�ѧ����ʽΪ ��
��2����ԭ�ϡ���Դ���õĽǶȣ�������Ӧ����Ϊ�ϳɼ״������˷�����ԭ���� ��
��3��ˮú���е�H2����������NH3���ڽ���ϳ���ǰ����[Cu(NH3)2]Ac��Һ���������е�CO����ֹ�ϳ����еĴ����ж����䷴Ӧ�ǣ� [Cu(NH3)2]Ac + CO + NH3  [Cu(NH3)3]Ac��CO ��H��0
[Cu(NH3)3]Ac��CO ��H��0
[Cu(NH3)2]Ac��Һ����CO��������������Ӧ�� ��
��4����CH4��Ƴ�ȼ�ϵ�أ��������ʸ��ߣ�װ��ʾ������ͼ��A��BΪ�����ʯī����������ͨ����飬�ڱ�״���£����ļ������VL��0��V��44.8 Lʱ������ܷ�Ӧ����ʽΪ ��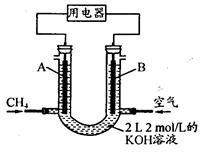
�� 44.8 L��V��89.6 Lʱ�������缫��ӦΪ ��
�� V="67.2" Lʱ����Һ������Ũ�ȴ�С��ϵΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����γɶ����������NO��NO2��N2O4�ȡ���֪NO2��N2O4�Ľṹʽ�ֱ���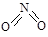 ��
��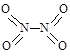 ��ʵ����N��N������Ϊ167kJ��mol��1�� NO2�е�������ƽ������Ϊ466 kJ��mol��1��N2O4�е�������ƽ������Ϊ438.5 kJ��mol��1��
��ʵ����N��N������Ϊ167kJ��mol��1�� NO2�е�������ƽ������Ϊ466 kJ��mol��1��N2O4�е�������ƽ������Ϊ438.5 kJ��mol��1��
��1��д��N2O4ת��ΪNO2���Ȼ�ѧ����ʽ��
��2���Է�ӦN2O4(g) 2NO2(g)�����¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ����
2NO2(g)�����¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ���� 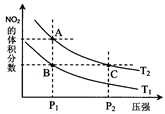
A��A��C����ķ�Ӧ���ʣ�A��C
B��B��C����������ƽ����Է���������B��C
C��A��C�����������ɫ��A�Cdz
D����״̬B��״̬A�������ü��ȵķ���
��3����100��ʱ����0.40mol��NO2�������2 L��յ��ܱ������У�ÿ��һ��ʱ��ͶԸ������ڵ����ʽ��з������õ����±����ݣ�
| ʱ�䣨s�� | 0 | 20 | 40 | 60 | 80 |
| n(NO2)/mol | 0.40 | n1 | 0.26 | n3 | n4 |
| n(N2O4)/mol | 0.00 | 0.050 | n2 | 0.080 | 0.080 |
 N2O4��ƽ�ⳣ��K�� �����������С�����䡱����
N2O4��ƽ�ⳣ��K�� �����������С�����䡱�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��15�֣����Ŵ�����Ⱦ���������أ��������ڡ�ʮ���塱�ڼ䣬����������(SO2)�ŷ�������8%����������(NOx)�ŷ�������10%��Ŀǰ������������Ⱦ�ж��ַ�����
(1)��CH4����ԭ��������������������������Ⱦ����֪��
��CH4(g)+4NO2(g)��4NO(g) + CO2(g) +2H2O(g) �SH�� -574 kJ��mol��1
��CH4(g) +4NO(g)��2N2(g) + CO2(g) + 2H2O(g) �SH��-1160 kJ��mol��1
��H2O(g)��H2O(l) ��H��-44.0 kJ��mol��1
д��CH4(g)��NO2(g)��Ӧ����N2 (g)��CO2 (g)��H2O(1)���Ȼ�ѧ����ʽ ��
��2������Fe2+��Fe3+�Ĵ����ã������¿ɽ�SO2ת��ΪSO42-���Ӷ�ʵ�ֶ�SO2����������֪��SO2�ķ���ͨ�뺬Fe2+��Fe3+����Һʱ������һ����Ӧ�����ӷ���ʽΪ4Fe2+ + O2+ 4H+ ��4Fe3+ + 2H2O������һ��Ӧ�����ӷ���ʽΪ ��
(3)�û���̿��ԭ��������������йط�ӦΪ��C(s)+2NO(g) N2(g)+CO2(g)��ij�о�С�����ܱյ���������У���������������䣬��������������Բ��ƣ�����NO�������Ļ���̿������(T1��)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
N2(g)+CO2(g)��ij�о�С�����ܱյ���������У���������������䣬��������������Բ��ƣ�����NO�������Ļ���̿������(T1��)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
Ũ��/mol��L��1
| NO | N2 | CO2 | ||
| 0 | 1.00 | 0 | 0 | ||
| 10 | 0.58 | 0.21 | 0.21 | ||
| 20 | 0.40 | 0.30 | 0.30 | ||
| 30 | 0.40 | 0.30 | 0.30 | ||
| 40 | 0.32 | 0.34 | 0.17 | ||
| 50 | 0.32 | 0.34 | 0.17 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�о�NO2��SO2 ��CO�ȴ�����Ⱦ����Ĵ���������Ҫ���塣
��1��NO2����ˮ���գ���Ӧ�Ļ�ѧ��Ӧ����ʽΪ ��
���÷�Ӧ6NO2�� 8NH3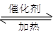 7N2��12 H2OҲ�ɴ���NO2����ת��1.2mol����ʱ�����ĵ�NO2�ڱ�״������ L��
7N2��12 H2OҲ�ɴ���NO2����ת��1.2mol����ʱ�����ĵ�NO2�ڱ�״������ L��
��2����֪��2SO2��g��+O2��g�� 2SO3��g�� ��H="-196.6" kJ·mol-1
2SO3��g�� ��H="-196.6" kJ·mol-1
2NO��g��+O2��g�� 2NO2��g�� ��H="-113.0" kJ·mol
2NO2��g�� ��H="-113.0" kJ·mol
��ӦNO2��g��+SO2��g�� SO3��g��+NO��g���Ħ�H= kJ·mol-1��
SO3��g��+NO��g���Ħ�H= kJ·mol-1��
һ�������£���NO2��SO2�������1:2�����ܱ������з���������Ӧ��������˵����Ӧ�ﵽƽ��״̬���� ��
a����ϵѹǿ���ֲ��� b�����������ɫ���ֲ���
c��SO3��NO������ȱ��ֲ��� d��ÿ����1 mol SO3��ͬʱ����1 molNO2
���¶��£��˷�Ӧ��ƽ�ⳣ������ʽK= ��
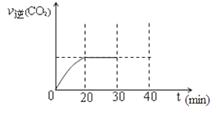
��3��CO�����ںϳɼ״�����Ӧ����ʽΪCO��g��+2H2��g�� CH3OH��g����CO�ڲ�ͬ�¶��µ�ƽ��ת������ѹǿ�Ĺ�ϵ����ͼ��ʾ���÷�Ӧ��H 0���>���� <������ʵ����������������250�桢1.3��104kPa���ң�ѡ���ѹǿ�������� ��
CH3OH��g����CO�ڲ�ͬ�¶��µ�ƽ��ת������ѹǿ�Ĺ�ϵ����ͼ��ʾ���÷�Ӧ��H 0���>���� <������ʵ����������������250�桢1.3��104kPa���ң�ѡ���ѹǿ�������� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com