
����Ŀ��ʵ��������240 mL1.0mol��L��1��NaOH��Һ��ʵ����������У�
A.���ձ�����ƽ�ϳƳ�ag�������ƹ��壬������������ˮʹ����ȫ�ܽⲢ��ȴ�����£�
B.����������ƿ�м�����ˮ��Һ���̶���1��2 cm�������ý�ͷ�ι�С�ĵμ�����ˮ����Һ����̶������У�
C.���Ƶõ���ҺС��ת��������ƿ�У�
D.������ƿƿ�����������ҡ�ȡ�
E.����������ˮϴ���ձ��Ͳ�����2��3�Σ�ÿ��ϴ�ӵ�Һ�嶼С��ע������ƿ��������
����д���пհף�
��1�������������ȷ˳��Ϊ_____������ĸ����
��2����ʵ������õ�����������ƽ��ҩ�ס����������ձ���____��____��
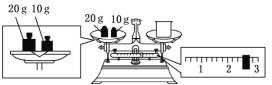
��3��ijͬѧ������NaOH��������������������ƽ�����ձ�����������ƽƽ����״̬��ͼ��ʾ������������ձ���ʵ������Ϊ___g��Ҫ��ɱ�ʵ���ͬѧӦ�Ƴ�___gNaOH��
��4��ʹ������ƿǰ������е�һ��������____��
��5�������ƹ����У���������������ȷ�ģ����в������������ƫ�ߵ���___��
A.����ֽ����NaOH����
B.����ʱ���ӿ̶���
C.δ��ȴ�����¾ͽ���Һת�Ƶ�����ƿ������
D.���ݺ�����ƿ��������תҡ�ȣ����ú�Һ����ڿ̶��ߣ��ټ�ˮ���̶���
��6����������ˮ����ʱ���������̶��ߣ�����������_____��
���𰸡�ACEBD 250mL����ƿ ��ͷ�ι� 27.4 10.0 ��© B C ��������
��������
(1)����ʵ������IJ��裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ����Լ�ÿ��������Ҫ����ȷ����Ӧ���������������(1)��(2)��
(3)����ͼʾ���ձ�������+2.6g=30g��ʵ��������240 mL1.0mol��L��1��NaOH��Һ����Ҫѡ��250mL����ƿ���ƣ��ݴ˷������
(4)����ƿ�ڲ������ӣ�ʹ��ǰ�����©��
(5)����c=![]() ��������ʵ����ʵ���n����Һ�����V�ı仯������������
��������ʵ����ʵ���n����Һ�����V�ı仯������������
(6)ʵ�����д�����Ҫ����������Һ��
(1)����240 mL 1.0mol��L��1��NaOH��Һ��һ�㲽��Ϊ�����㡢�������ܽ⡢��ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȣ����Բ����������ȷ˳��Ϊ��ACEBD���ʴ�Ϊ��ACEBD��
(2)ʵ��IJ��������У����㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ�ù���ҩƷ�����ձ����ܽ⣬��ȴ��ת�Ƶ�250mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ�����������250mL����ƿ����ͷ�ιܣ��ʴ�Ϊ��250mL����ƿ����ͷ�ιܣ�
(3)����ͼʾ���ձ�������+2.6g=30g�����ձ�������Ϊ27.4g��ʵ��������240 mL1.0mol��L��1��NaOH��Һ����Ҫѡ��250mL����ƿ������250 mL��Һ����ҪNaOH������=0.25L��1.0mol��L��1��40g/mol=10.0g���ʴ�Ϊ��27.4��10.0��
(4)�����ӵ�����ʹ��ǰҪ�Ȳ�©��������ƿʹ��ǰ�����©���ʴ�Ϊ����©��
(5)A���������ƹ������׳��⣬����ֽ����NaOH���壬ʹ���������Ƶ�����ƫС�������Ƶ���Һ��Ũ��ƫ�ͣ���A����
B������ʱ���ӿ̶��ߣ��ᵼ����Һ���ƫС����Ũ��ƫ�ߣ���B��ȷ��
C��δ��ȴ�����¾ͽ���Һת�Ƶ�����ƿ�����ݣ������ȴ����Һ���ƫС��Ũ��ƫ�ߣ���C��ȷ��
D�����ݺ�����ƿ������ҡ�ȣ����ú�Һ����ڿ̶����������ģ��ټ�ˮ���̶��ᵼ��Ũ��ƫ�ͣ���D����ѡBC��
(6)��������ˮ����ʱ���������̶��ߣ���ȷ�Ĵ��������ǣ�������ƿϴ�Ӹɾ����������ƣ��ʴ�Ϊ���������ơ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ijԪ�صļ����άͼ������AΪ���Σ�X��һ��ǿ�ͨ��������Z����ɫҺ�壬E����Է���������D��16�������ʵ�ת����ϵ��ͼ��ʾ������˵���������
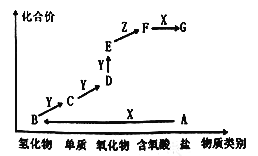
A. A������ʱ���ʺ����ľ�һ��ʩ��
B. ͬ����Ԫ�ص��⻯����B�ķе����
C. Cһ������ˮ���ռ�
D. D��E�ķ�Ӧ�����ڼ���D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������Ҫ����0.50 mol��L��1NaCl��Һ480 mL�������в������������ʵ������֣���ʹ��������������
��1��ѡ����������ɱ�ʵ��������������У�������ƽ(�����롢��С����Ϊ5 g)��ҩ�ס��ձ���________��________��________�Լ�����������Ƭ��ֽ��
��2�����㡣���Ƹ���Һ��ȡNaCl����________g��
��3��������
����ƽ��ƽ֮��Ӧ����ƽ���������ij��λ�ã�������ͼ����һ�����߱���������Ե������λ�ã�________
![]()
�ڳ���������NaCl����Ӧ������ƽ��________(��������������������)��
�۳�����ϣ���ҩƷ�����ձ��С�
��4���ܽ⡢��ȴ���ò�ʵ������Ҫʹ�ò�������Ŀ����___________________��
��5��ת�ơ�ϴ�ӡ���ת��ʱӦʹ�ò�������������Ҫϴ���ձ�2��3����Ϊ��________________��
��6�����ݡ�������ƿ�м�ˮ��Һ��ӽ��̶���________��������________��ˮ��ʹ��Һ��Һ����̶������С�
��7��ҡ�ȡ�װƿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������ͨ��������ˮ�Ҵ���Ũ����Ļ����ķ�����ȡ��ϩ���ھ���Ļ�ѧʵ���г�����ȵ��¶ȹ��ߵ��¸���Ӧ������ʹ��ϩ�����л��ж����������壬�����������ʵ����ȷ����ϩ�����л��ж����������塣��ش��������⣺
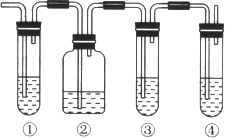
(1) ͼ�Тٺܵ͢�������ʢ�ŵĻ�ѧ�Լ��ֱ���(��д����)
��_____________________����_________________________��
A��Ʒ����Һ�� B���ռ���Һ�� C��Ũ���ᡡ D�����Ը��������Һ
(2)��˵����������������ڵ�������____________________________________________��
(3)ʹ��װ�âڵ�Ŀ����______________________��ʹ��װ�â۵�Ŀ����__________________��
(4)ȷ����ϩ������ڵ�������_____________________________________________________��
(5)ȷ����ϩ����Ĵ��ڻ�������ˮ����д����ϩ����ˮ��Ӧ�ķ���ʽ_________________���䷴Ӧ��Ϊ______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵������ȷ���ǣ� ��
A.��1L2mol��L-1��NaCl��Һ��ȡ��10mL��������Һ��Ũ����0.02mol��L-1
B.��״����22.4LHCl��������1Lˮ��������Һ��Ũ��Ϊ1.00mol��L-1
C.98��(�ܶ�1.84g��cm-3����������ʵ���Ũ����18.4mol��L-1
D.10mL10.0mol��L-1 Na2SO4��Һ��ˮ90mL��������Һ��Ũ����1.00mol��L-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ۻ� (As4S4)�ʹƻ�(As2S3)����ȡ�����Ҫ����ԭ�ϣ���������Ȼ���й����������������������գ�
��1�� As2S3��SnCl2�������з�Ӧת��ΪAs4S4��SnCl4���ų�H2S���塣��As2S3��SnCl2������ȫ��Ӧ��As2S3��SnCl2�����ʵ���֮��Ϊ______��
��2��������Ӧ�е���������_____����Ӧ�������������____���ա�
��3��As2S3��HNO3�����·�Ӧ��As2S3 + 10H+ + 10NO3- = 2H3AsO4+ 3S+10NO2��+ 2H2O��������2mol H3AsO4����Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ____��
��4������Ӧ����NO2��11.2L O2����״������Ϻ���ˮ����ȫ��ת����ŨHNO3��Ȼ���������̼��Ӧ����������CO2����_____������ĸ����
a��С��0.5 mol b������0.5 mol c������0.5mol d����ȷ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Na��ʾ�����ӵ�������ֵ�����������������
A.����NA����ԭ�ӵ������ڱ�״���µ����ԼΪ11.2L
B.25����1.01��105Pa��64gSO2�к��е�ԭ����Ϊ3Na
C.�ڳ��³�ѹ�£�35.5g Cl2���еķ�����Ϊ0.5Na
D.��״���£�11.2LH2O���еķ�����Ϊ0.5NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪A��B��C��D��E�ǻ�ѧ�г��������ʣ������£�E��һ����ɫ��ζ��Һ�壬����֮�������·�Ӧ��ϵ��
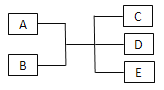
��1����A��һ�ֺ�ɫ�������ʣ�B��һ�ֳ����Ļӷ����ᣬ��Ӧʱ���ɵ�C����ɫ���壬��Ӧ�����ӷ���ʽΪ___________������Ӧ�ų�1.12 L���壨��״���£�����ԭ��B���ʵ���Ϊ___________mol��
��2����ʵ���������ù���A��B�ķ�Ӧ�Ʊ�����C��C��һ����ɫ���̼�����ζ���ܶȱȿ���С�����Ե����壬��д���˷�Ӧ�Ļ�ѧ����ʽ________________��ʵ���Ҽ���C�ķ���Ϊ_______________��
��3����B�ǻ���ɫ�ж����壬������ϵ��������ʵ����β����������Ӧ�����ӷ���ʽΪ________________������ʪ��ĵ��۵⻯����ֽ����ʢ��B���Թܿڣ�����������Ϊ______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������Ҫ����0.50 mol��L��1 NaOH��Һ480 mL�������в������������ʵ������֣���ʹ��������������
��1��ѡ����������ɱ�ʵ��������������У�������ƽ(��ȷ��0.1 g)��ҩ�ס��ձ�����������________��________��
��2�����㡣���Ƹ���Һ���ȡNaOH���������Ϊ________g��
��3��������
��4���ܽ⡢��ȴ��
��5��ת�ơ�ϴ�ӡ�
��ʹ������ƿǰ����________________������ƿ��ע������⣬������_______��ѡ���ţ���
a.�¶� b.Ũ�� c.ѹǿ d.�̶��ߣ�
����ת��ʱӦʹ�ò�����������ϴ���ձ�2��3����Ϊ��_________________________��
��6�����ݣ�ҡ�ȡ�
��7������ȷ���������������Һ���ʵ���Ũ��Ϊ0.192mol/L,ԭ�������___________��
A.ʹ����ֽ����NaOH���壻
B.�ܽ�NaOH����ձ�δ�����ϴ�ӣ�
C.����ƿ��ԭ������������ˮ��
D.����ʱ���õ��������⣻
E.δ��ȴֱ��ת��������ƿ��������ã�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com