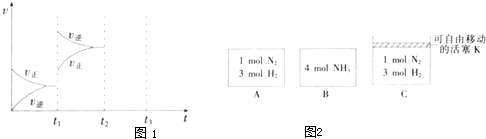
��2012?ʯ��ׯһģ��ij��ѧС��Ϊ�ⶨij����������Fe
xS
y����ɣ��������²��������ʵ�飮
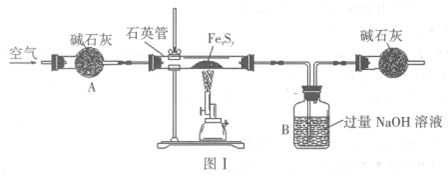
����һ������ͼ����ʾװ�ã���������4.16g Fe
xS
y�������������ٱ仯���õ�����ɫ�����һ����ʹƷ����Һ��ɫ�����壮
���������B��������Һ��ͼ����д�����
��ش�
��1������һ�У���FexS
y������ȫ��Ӧ�������ͨ�����Ƭ�̣���Ŀ��Ϊ
�����ɵ�SO2����ȫ������B�б�NaOH��Һ��ȫ���գ���ֹB��Һ�嵹����Ӳ�ʲ������У�
�����ɵ�SO2����ȫ������B�б�NaOH��Һ��ȫ���գ���ֹB��Һ�嵹����Ӳ�ʲ������У�
��2������H
2O
2��Һ������Ϊ
SO32-+H2O2�TSO42-+H2O
SO32-+H2O2�TSO42-+H2O
�������ӷ���ʽ��ʾ����
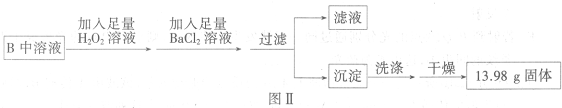
��3������ʱ�õ��IJ����������ձ��⣬����
©����������
©����������
�����������ƣ���
��4���жϲ�����г����Ƿ��Ѿ�ϴ�Ӹɾ��IJ�������Ϊ
ȡ���һ��ϴ�����õ���Һ�������Թ��У��μ�ϡ���ᣨ�����ƻ������ữ����������Һ�������ް�ɫ�������ɣ�˵��������ϴ�Ӹɾ������а�ɫ�������ɣ�˵������δϴ�Ӹɾ�
ȡ���һ��ϴ�����õ���Һ�������Թ��У��μ�ϡ���ᣨ�����ƻ������ữ����������Һ�������ް�ɫ�������ɣ�˵��������ϴ�Ӹɾ������а�ɫ�������ɣ�˵������δϴ�Ӹɾ�
��
��5�����������ṩ�����ݿɼ���ó�����������Ļ�ѧʽΪ
Fe2S3
Fe2S3
��
��6��ʵ�����������װ��A��������Fe
xS
y��x��y��ֵ��
ƫС
ƫС
���ƫ����ƫС������Ӱ�족����
��7��ijͬѧ��Ϊ���ý��в������ֻ��ͨ���ⶨ����һ��װ��B�е���Һ�ڷ�Ӧǰ������������ȷ��x��y��ֵ��ʵ��֤�����˷����ⶨ�Ľ��ƫС����ԭ��Ϊ
��������������װ��B����SO32-��Ӧ����SO42-�����B����Һ�ڷ�Ӧǰ�������������
��������������װ��B����SO32-��Ӧ����SO42-�����B����Һ�ڷ�Ӧǰ�������������
��



 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�