
����Ŀ����1���±��ո��е�������ʽΪ_______������_______��ͬ���칹�塣
1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
CH4 | C2H4 | C3H8 | C4H8 | C6H12 | C7H16 | C8H16 |
��2��������(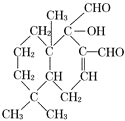 )��һ������ɱ������京�������ŵ�����Ϊ_____________________��
)��һ������ɱ������京�������ŵ�����Ϊ_____________________��
��3��CH2=CH-![]() -C��C-CH3�У������_______��ԭ�ӹ��档
-C��C-CH3�У������_______��ԭ�ӹ��档
��4���ٸ���������д�ṹ��ʽ��2-��-2,4-�Ѷ�ϩ_________________________________
��5���л���![]() ��ϵͳ������___________________________________�������ڴ�����������ȫ�⻯������������ϵͳ������_________________________________
��ϵͳ������___________________________________�������ڴ�����������ȫ�⻯������������ϵͳ������_________________________________
��6��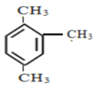 ��ϵͳ������______________________________
��ϵͳ������______________________________
���𰸡� C5H12 3 �ǻ� ȩ�� 19 ![]() 5��6����3�һ�1��Ȳ 2��3����5�һ����� 1,2,4-������
5��6����3�һ�1��Ȳ 2��3����5�һ����� 1,2,4-������
����������1�����ݱ����й��ɿ�����������ϩ����˳����֣���������ϩ����һ��̼�����Ե���5λʱΪ���飬����ʽΪC5H12��������3��ͬ���칹�壺�����顢������������飻��ȷ�𰸣�C5H12 ��3��
��2���ӻ�����(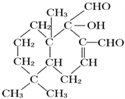 )�ṹ�������京�������ŵ�����Ϊ�ǻ��� ȩ������ȷ�����ǻ��� ȩ����
)�ṹ�������京�������ŵ�����Ϊ�ǻ��� ȩ������ȷ�����ǻ��� ȩ����
��3����ϩ��Ϊƽ��ṹ��5��ԭ�ӹ�ƽ��������Ϊƽ��ṹ��![]() ����6��̼+4�������ƽ�棻��Ȳ��Ϊֱ�߽ṹ������-C��C-��2��̼��ֱ����Ҳ�ɺͱ�����ƽ����-CH3�������1��̼��1����������ԭ�ӹ�ƽ�������CH2=CH-
����6��̼+4�������ƽ�棻��Ȳ��Ϊֱ�߽ṹ������-C��C-��2��̼��ֱ����Ҳ�ɺͱ�����ƽ����-CH3�������1��̼��1����������ԭ�ӹ�ƽ�������CH2=CH-![]() -C��C-CH3�У������5+6+4+2+2=19��ԭ�ӹ��棻��ȷ�𰸣�19��
-C��C-CH3�У������5+6+4+2+2=19��ԭ�ӹ��棻��ȷ�𰸣�19��
��4������̼��Ϊ6��̼��2��̼��1������̼̼˫���ֱ�Ϊ2�š�4��̼�ϣ�����2-��-2,4-�Ѷ�ϩ�ṹ��ʽΪ![]() ����ȷ�𰸣�
����ȷ�𰸣�![]() ��
��
��5���л���![]() ����Ȳ��������̼̼�������ڵ���̼��Ϊ7��̼��̼̼������1��̼�ϣ�����5,6��̼�ϣ��һ���3��̼�ϣ����л��������Ϊ5��6-����-3-�һ�1��Ȳ�������ڴ�����������ȫ�⻯��õ���������̼��Ϊ7��̼��2,3��̼���м���5��̼�����һ������л��������Ϊ2��3-����-5-�һ����飻��ȷ�𰸣�5��6-����-3-�һ�1��Ȳ��2��3-����-5-�һ����顣
����Ȳ��������̼̼�������ڵ���̼��Ϊ7��̼��̼̼������1��̼�ϣ�����5,6��̼�ϣ��һ���3��̼�ϣ����л��������Ϊ5��6-����-3-�һ�1��Ȳ�������ڴ�����������ȫ�⻯��õ���������̼��Ϊ7��̼��2,3��̼���м���5��̼�����һ������л��������Ϊ2��3-����-5-�һ����飻��ȷ�𰸣�5��6-����-3-�һ�1��Ȳ��2��3-����-5-�һ����顣
��6��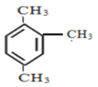 ���л���Ϊ����������������ԭ�����Ϸ��ļ���ʼ���Ϊ1��˳ʱ����б�ţ��ڶ�����Ϊ2����������Ϊ4�����Ը��л��������Ϊ1��2��4-����������ȷ�𰸣�1��2��4-��������
���л���Ϊ����������������ԭ�����Ϸ��ļ���ʼ���Ϊ1��˳ʱ����б�ţ��ڶ�����Ϊ2����������Ϊ4�����Ը��л��������Ϊ1��2��4-����������ȷ�𰸣�1��2��4-��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������һ����Ҫ�Ļ���ԭ�ϣ���ҵ�Ͽ�ͨ������;���Ʊ�:

��ش���������:
(1)�����ϩ�����������________��ԭ�ӹ�ƽ��;
(2)�����ķ���ʽΪ_____��������е�-CH2-������_____��;
(3)��֪ϩ���ܷ������·�Ӧ:
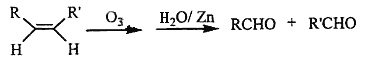 ��
��
��д�����з�Ӧ����Ľṹ��ʽ:
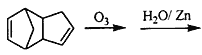 ��_______
��_______
(4)A�Ƕ��ۻ����ϩ��ͬ���칹�壬��ʹ������Ȼ�̼��Һ��ɫ��A���������������Һ�����������Եõ��Ա�������[��ʾ: �����ϵ����(-CH3��-CH2R��-CHR2)��ϩ���������������������Һ�������Ȼ�]��д��A���п��ܵĽṹ��ʽ(�����������칹):_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��NA���������ӵ�������ֵ������˵����ȷ����
A. ���³�ѹ�£�124 g P4������P��P����ĿΪ4NA
B. 100 mL 1mol��L1FeCl3��Һ������Fe3+����ĿΪ0.1NA
C. ��״���£�11.2 L�������ϩ������к���ԭ����ĿΪ2NA
D. �ܱ������У�2 mol SO2��1 mol O2����Ӧ���������Ϊ2NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��һ���绯ѧ���̵�ʾ��ͼ���ش��������⣺

��1���׳ؽ�______��ת��Ϊ______�ܣ���װ���е缫A��____����
��2����װ����ͨ��CH4�ĵ缫��ӦʽΪ__________________________________����װ���е缫B(Ag)�ĵ缫��ӦʽΪ________________________________��
��3��һ��ʱ�䣬�������в���112 mL(��״��)����ʱ�����Ƚ�����أ�������Һ��25 ��ʱ��pH��________(��֪��NaCl��Һ������������Һ���Ϊ500 mL)����Ҫʹ���ػָ����ǰ��״̬��Ӧ�������ͨ��________(д��ѧʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2.8g Feȫ������һ��Ũ�ȡ�200mL��HNO3��Һ�У��õ���״���µ�����1.12L����÷�Ӧ����Һ��pHΪ1������Ӧǰ����Һ����仯���Բ��ƣ������й��ж���ȷ����
A. ��Ӧ����Һ��c��NO3����=0.85mol/L
B. ��Ӧ�����Һ�����ܽ�1.4gFe
C. ��Ӧ����Һ����Ԫ�ؿ�����Fe2+��ʽ����
D. 1.12L���������NO��NO2�Ļ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��AΪ278g/mol��B��D��E��F��G����������BΪ����ɫ���壬F��K���⻯�C��H���ճ�����������Ľ������ʣ�J�ǻ���ɫ���塣O�ǰ�ɫ������ͼ�в��ַ�Ӧ���������û���г�����

��1��д��A��G��L�Ļ�ѧʽA______________��G�� _______________��L��______________��
��2����Ӧ�ڵĻ�ѧ����ʽ ________________________________________________��
��3��д����ӦM��L�����ӷ���ʽΪ________________________________________��
��4������O���ھ��ã����ʹ����е�����Ϊ___________________________________�������Ļ�ѧ����ʽΪ__________________________________________________��
��5����M��Һ��Ͷ����M�����ʵ�����Na2O2����Ӧ�����ӷ���ʽ��____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������У�����������ѧ��Ӧ��ʹ��ˮ��ɫ������ʹ�������������Һ��ɫ����( )
��CH3CH2CH2CH3 ��CH3CH2CH===CH2

A���٢ڢۢ� B���ڢۢ�
C���ڢ� D��ֻ�Т�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����NaCl��Һ�еμ�AgNO3��Һ������Ϊ_____________________________ �� ���ӷ���ʽΪ:_________________________________________________
��2����CH3CH2CH2Cl�еμ�AgNO3��Һ������Ϊ_____________________________��ԭ����__________________________
��3�����Ƚ�CH3CH2CH2Cl��NaOH��Һ���ȣ�Ȼ���������ữ���ٵμ�AgNO3��Һ������Ϊ_______________________________________________________����Ӧ�Ļ�ѧ����ʽΪ:________________________��______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D����ѧ��ѧ�еij������ʣ������ʵ�ת����ϵ���£�
![]()
(1)��A�ǻ�ɫ���壬B���γ��������Ҫ����֮һ��
������A��ԭ�ӽṹʾ��ͼ____________��
�� д��D��Ũ��Һ�����е���������________________(���ּ���)��
(2)���˷�Ӧ�����ܽ���ũ�������귢ׯ�����Ļ�ѧԭ����������������ȷ����________��
a.A�Ļ�ѧ���ʽ�Ϊ�ȶ� b.B��C��Ϊ����������
c.�����еķ�Ӧ����������ԭ��Ӧ d.����D�������Ӧ��ȡ����
(3)��A��B��C��D����ɫ��Ӧ��Ϊ��ɫ��CΪ����ɫ���壬A�ڼ��������¿�ֱ��ת��ΪC���� C��D�Ļ�ѧ����ʽ��__________��
(4)��A�ǻ�����Ҵ����̱�ʾ��ҵ�����������ת����ϵ����A��B�ķ�Ӧ����ʽΪ_______________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com