
����Ŀ����ͼ��AΪ278g/mol��B��D��E��F��G����������BΪ����ɫ���壬F��K���⻯�C��H���ճ�����������Ľ������ʣ�J�ǻ���ɫ���塣O�ǰ�ɫ������ͼ�в��ַ�Ӧ���������û���г�����

��1��д��A��G��L�Ļ�ѧʽA______________��G�� _______________��L��______________��
��2����Ӧ�ڵĻ�ѧ����ʽ ________________________________________________��
��3��д����ӦM��L�����ӷ���ʽΪ________________________________________��
��4������O���ھ��ã����ʹ����е�����Ϊ___________________________________�������Ļ�ѧ����ʽΪ__________________________________________________��
��5����M��Һ��Ͷ����M�����ʵ�����Na2O2����Ӧ�����ӷ���ʽ��____________��
���𰸡�FeSO4��7H2OAl2O3FeCl3SO2+Cl2+2H2O=4H2SO4+2HCl2Fe2++Cl2=2Fe3++2Cl-��ɫ�����ɫ����ɫ4Fe(OH)2+O2+2H2O=4 Fe(OH)34Na2O2 + 4Fe2+ + 6H2O = 8Na+ + O2�� + 4 Fe(OH)3��
��������
ץסB��D��E��F��G�������F��K���⻯�C��H���ճ�����������Ľ������ʣ�B + C��G + H��Ӧ�������Ǹ��£��������뵽�����ȷ�Ӧ��C���� G����������K + H ��M��K���⻯�O�ǰ�ɫ������B��H��L��M��N��O�к���ͬ��Ԫ�أ��Լ�J+H��L��M+J��L��L��N��M��O��O��N��N��B���ݹ�ϵ���Զ϶�B��H��L��M��N��O�к���ͬ��Ԫ����Fe ����DΪ��������EΪ��������FΪˮ��HΪ����IΪ���ᡢJΪ������KΪ���ᡢLΪ�Ȼ�����MΪ�Ȼ�������OΪ������������NΪ����������BΪ����ɫ������Ϊ��������AΪ278g/mol�ۺϷ���֪��A����ˮ���������������Ԣ�A��G��L�Ļ�ѧʽA��FeSO4��7H2O��G��Al2O3��L��FeCl3���Ʒ�Ӧ���Ƕ���������������ˮ��Ӧ������������ᣬ��Ӧ�����ӷ���ʽΪSO2+Cl2+2H2O=4H2SO4+2HCl���Ƿ�ӦM��L���Ȼ�������������Ӧ�����Ȼ�������Ӧ�����ӷ���ʽΪ2Fe2++Cl2=2Fe3++2Cl-����OΪ���������������ھ��ã������ᱻ�����е��������������������������������������ʱ��ʹ����е�����Ϊ��ɫ�����ɫ����ɫ�������Ļ�ѧ����ʽΪ4Fe(OH)2+O2+2H2O=4 Fe(OH)3����5��MΪ�Ȼ���������M��Һ��Ͷ����M�����ʵ�����Na2O2����Ӧ�����ӷ���ʽ��4Na2O2 + 4Fe2+ + 6H2O = 8Na+ + O2�� + 4 Fe(OH)3����


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ĿҪ��ش�����
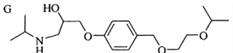
��1��д�������ʵķ���ʽΪ___________д�����л����к��������ŵ�����_________
��2����֪ij�л���Ľṹ��ʽ��ͼ��ʾ��д��ͬʱ������������Ҫ���ͬ���칹��Ľṹ��ʽ��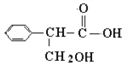
�ٻ�������1,3,5-��ȡ����
�ڱ����ϵ�����ȡ�����ֱ��Ǽ����ǻ��ͺ��������ṹ�Ļ��š�
____________________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ֲ��֦Ҷ��ȡ�ľ����к��мס������ֳɷ�:
![]()
��1�����к������ŵ�����Ϊ_______��______________��
��2���ɼ�ת��Ϊ�ҵĹ���Ϊ(����ȥ�ز���):
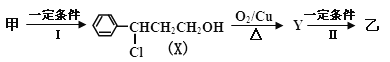
�ٷ�ӦII �ķ�Ӧ����Ϊ____________��Y�Ľṹ��ʽ___________________��
����Ʋ���I��Ŀ����___________________________________________________________��
��3����˳��д������X��±��ԭ��������Լ�________________________________��
��4��д����������Cu (OH)2����Һ��Ӧ�Ļ�ѧ����ʽ______________________________��
��5�� �Ҿ��������õ���(![]() )д��ͬʱ��������Ҫ��ı���ͬ���칹��Ľṹ��ʽ_____________________��_______________________��
)д��ͬʱ��������Ҫ��ı���ͬ���칹��Ľṹ��ʽ_____________________��_______________________��
�� �ܷ���������Ӧ����FeCl3��Һ��ɫ�ۺ˴Ź���������ɫ��4 ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڸ��������£�����ѡ����ʾ�����ʼ�ת������ʵ�ֵ���
A. NaHCO3(s)![]() Na2CO3(s)
Na2CO3(s)![]() NaOH(aq)
NaOH(aq)
B. Al(s)![]() NaAlO2(aq)
NaAlO2(aq)![]() Al(OH)3(s)
Al(OH)3(s)
C. AgNO3(aq)![]() [Ag(NH3)2]+(aq)
[Ag(NH3)2]+(aq)![]() Ag(s)
Ag(s)
D. Fe2O3(s)![]() Fe(s)
Fe(s)![]() FeCl3(aq)
FeCl3(aq)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1���±��ո��е�������ʽΪ_______������_______��ͬ���칹�塣
1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
CH4 | C2H4 | C3H8 | C4H8 | C6H12 | C7H16 | C8H16 |
��2��������(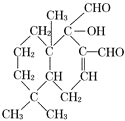 )��һ������ɱ������京�������ŵ�����Ϊ_____________________��
)��һ������ɱ������京�������ŵ�����Ϊ_____________________��
��3��CH2=CH-![]() -C��C-CH3�У������_______��ԭ�ӹ��档
-C��C-CH3�У������_______��ԭ�ӹ��档
��4���ٸ���������д�ṹ��ʽ��2-��-2,4-�Ѷ�ϩ_________________________________
��5���л���![]() ��ϵͳ������___________________________________�������ڴ�����������ȫ�⻯������������ϵͳ������_________________________________
��ϵͳ������___________________________________�������ڴ�����������ȫ�⻯������������ϵͳ������_________________________________
��6��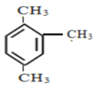 ��ϵͳ������______________________________
��ϵͳ������______________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��ȡ3.7gij�л���A�����������г��ȼ�գ�ֻ����8.8 gCO2��4.5g H2O����A�к��е�Ԫ��Ϊ_________________����Ԫ�ط��ţ�����ʵ��ʽΪ____________��
��2����ͼ��A������ͼ��������Է�������Ϊ ________ ������ʽΪ _________��
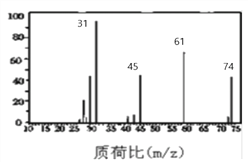
��3�����ⶨ��A�ں˴Ź��������г����ĸ��壬���շ����֮��Ϊ6��1��2��1��A������Ʒ�Ӧ������������A�Ľṹ��ʽΪ ____________________________��
��4��A�ж���ͬ���칹�壬д����A������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ����д���֣���_________________________��_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ���� ( )
A.�������ָ���κ������¾��ܵ���Ļ�����
B.NH3��CO2��ˮ��Һ���ܵ��磬����NH3��CO2���ǵ����
C.���ǡ��ƾ���Һ̬��ˮ��Һ��������磬���������Ƿǵ����
D.ͭ��ʯī�����磬���������ǵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������Ϊ10 mL�����ʵ���Ũ����ͬ������NaOH ��Һ�зֱ�ͨ��һ������CO2���õ���Һ���ҡ���ס�������Һ�зֱ�μ�0.1mol/L����������ʱ��Ӧ����CO2���(��״��)��������������Ĺ�ϵ��ͼ��ʾ�������������в���ȷ����

A. ԭNaOH��Һ�����ʵ���Ũ��Ϊ0.5 mol/L
B. ��0����)<10 mLʱ������Һ�з�����Ӧ�����ӷ���ʽΪH++CO32-=HCO3-
C. ����Һ�к��е�������Na2CO3��NaOH
D. ������Һ�еμӹ�����������CO2��������ֵΪ224 mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и������ӣ�������Һ�д����������
A. Fe2����Ba2����H+��NO3�� B. Ba2����Na����CO32���� OH��
C. K����Mg2+��SO42����Cl�� D. NH4+��Na+��NO3����OH��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com