
����Ŀ���ڶ������Ƿǽ���Ԫ���������ڣ��ش��������⡣
(1)����������У�Bԭ����3���ǻ��������侧�������ʯī���ƵIJ�״�ṹ����ͬ����Ӽ����Ҫ��������___________________
(2)��һ�����ܽ���B��N֮��ĵڶ�����Ԫ����_________________�֡�
(3)HNO3�����Ա�HNO2ǿ���Դӽṹ�ĽǶȽ�����ԭ��_____________________
(4)̼���ͬ���壬�����Ľṹͬ���ʯ��ͬ���Ʋ����۵�Ƚ��ʯ��________���ߡ��ͣ������ԭ�ӵİ뾶Ϊr����辧�徧�����ⳤΪ_______________���ô�r����ʽ��ʾ���������Ŀռ�������Ϊ______________��������λ��Ч���֣�
(5)ʯīϩ�����R�����γ�һ�ֲ�㻯�������R��ƽ����ʯī�㣬������ͼ����ʾ���䴹ֱ��ʯī�㷽���ͶӰ��ͼ����ʾ����ò�㻯����Ļ�ѧʽΪ_____��

���𰸡���� 3 HNO3�з��ǻ��������� �� ![]() 34% RC8
34% RC8
��������
��1��������[B��OH��3]�����У���ԭ������ԭ���γ�1�Թ��õ��Ӷԣ���Ԫ�صĵ縺�Ժ�ǿ����ͬ��������е���ԭ������ԭ��֮���γ�����������������֮����ڷ��»���������ȷ��»�����ǿ��
��2����һ�����ܽ���B��N֮��ĵڶ�����Ԫ����Be��C��O����Ԫ�أ�
��3�����ǻ��������࣬����ԭ���ĵ���������ƫ���Խ�������Ӷ��������ĵ�����ͨ����������ƫ���Խ�������Ӷ��������룬����Խǿ��
��4�����е��ߵͿ�����������Խ�̣�����Խ���۷е�Խ�ߣ�
��5���������ͼ�ס�ͼ�ҿɵã�������Rԭ����Ŀ=8��1/8+6��1/2+4=8��Cԭ����Ŀ=12��4+8��4��1/2=64�����Ըò�㻯����Ļ�ѧʽΪRC8��
��1��������[B��OH��3]�����У���ԭ������ԭ���γ�1�Թ��õ��Ӷԣ���Ԫ�صĵ縺�Ժ�ǿ����ͬ��������е���ԭ������ԭ��֮���γ�����������������֮����ڷ��»���������ȷ��»�����ǿ�����������֮����Ҫ��������ʴ�Ϊ�������
��2��ͬһ����Ԫ���У�Ԫ�صĵ�һ����������ԭ����������������������ƣ����ڢ�A�塢�ڢ�A��Ԫ��ԭ�Ӵ���ȫ��������������ȶ�״̬���������һ�����ܴ���������Ԫ�أ��ʵ�һ�����ܽ���B��N֮��ĵڶ�����Ԫ����Be��C��O����Ԫ�أ��ʴ�Ϊ��3��
��3������ͬ��Ԫ��R�IJ�ͬ�������������ԱȽϣ�����������ķ���������д��(HO)nROm����ʽ��mԽ�������Խǿ��mԽ���ǻ��������࣬����ԭ���ĵ���������ƫ���Խ�������Ӷ��������ĵ�����ͨ����������ƫ���Խ�������Ӷ��������룬����Խǿ���ʴ�Ϊ��HNO3�з��ǻ��������ࡣ
��4�������ͽ��ʯ����ԭ�Ӿ������������е��ߵͿ�����������Խ�̣�����Խ���۷е�Խ�ߣ����ʯ��C-C�����������Si-Si������ΪC��ԭ�Ӱ뾶С��Si������C-C������С��Si-Si�������������۵㣺���ʯ>����裻�������֪�������Ľṹͬ���ʯ��ͬ���þ����к��й�ԭ�ӵĸ���Ϊ8����8����ԭ�ӵ����Ϊ8��4/3��r3pm3����ù辧�徧�����ⳤΪapm������2r=![]() /4a�����a=8/3
/4a�����a=8/3![]() rpm�����Ծ��������Ϊ��8/3
rpm�����Ծ��������Ϊ��8/3![]() r��3pm3�����Ŀռ�������Ϊ8��4/3��r3����8/3
r��3pm3�����Ŀռ�������Ϊ8��4/3��r3����8/3![]() r��3��100%=34%���ʴ�Ϊ���ͣ�
r��3��100%=34%���ʴ�Ϊ���ͣ�![]() ��34%��
��34%��
��5���������ͼ�ס�ͼ�ҿɵã�������Rԭ����Ŀ=8��1/8+6��1/2+4=8��Cԭ����Ŀ=12��4+8��4��1/2=64�����Ըò�㻯����Ļ�ѧʽΪRC8���ʴ�Ϊ��RC8��


 �ظ���ʦ�㲦ϵ�д�
�ظ���ʦ�㲦ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʵ�����Ʊ���������֤�������ʵ�װ��(���мг�װ����ʡ��)��
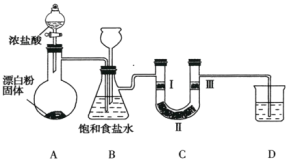
��֪��װ��A�������ķ���װ�ã���Ӧ�Ļ�ѧ����ʽΪ![]() ��
��
�ݴ˻ش��������⣺
(1)װ��B�б���ʳ��ˮ��������_________��
(2)װ��BҲ�ǰ�ȫƿ��Ŀ���Ǽ��ʵ�����ʱװ��C���Ƿ�����������д��װ��C�з�������ʱװ��B�е�ʵ������__________________��
(3)װ��C����������֤�����Ƿ����Ư���ԣ���װ��C��I��������Ӧ�����������___________(����ĸ)��
��� | I | �� | �� |
a | �������ɫ���� | ��ʯ�� | ʪ�����ɫ���� |
b | �������ɫ���� | ��ˮ����ͭ | ʪ�����ɫ���� |
c | ʪ�����ɫ���� | Ũ���� | �������ɫ���� |
d | ʪ�����ɫ���� | ��ˮ�Ȼ��� | �������ɫ���� |
(4)װ��D��������_____________________________��
(5)�����20mL��10mol��L-1��Ũ����������������Ƴ�ַ�Ӧ��ʵ�����ռ����������ڱ�״���µ������__________��
A.��2.24 L B.��2.24 L C.��2.24 L D.��2.24 L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ϩ������װ���ϵ����ԭ�ϣ�ʧ��ʱ������ϩ�ڲ�ͬ���¶��£�����һϵ�и��ӵĻ�ѧ�仯�����������к����壬����̴������£�

����˵������ȷ����
A. ������ϩ�ĵ��������ϩ��HCl�ӳɶ���
B. ������Ӧ�Т�������ȥ��Ӧ�������ڣ����⣩������Ӧ
C. �������ɾ�����ϩ�������к������к�HCl��CO��C6H6��
D. �ڻ����ֳ���������ʪë����ס�ڱǣ�������������Զ���ֳ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ֶ�����Ԫ�������ڱ��е�λ����ͼ������MΪ�ǽ���Ԫ��![]() ����˵����ȷ����
����˵����ȷ����
![]()
A. M�����������Ϊһ������
B. Z�����Ӱ뾶��ͬ���ڸ�����������С��
C. X����̬�⻯����ȶ��Ա�Y��ǿ
D. Z���⻯��������ͬ������ǿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������ˮʱ�ᷢ��������������ƶ������ӣ��絼�ʱ仯һ���̶��Ͽ��Է�ӳ��Һ�������ƶ�������Ũ�ȱ仯����Һ�������ƶ�������Ũ��Խ�絼�ʾ�Խ����ͼ����20mL0.01mol/LBa(OH)2��Һ�е�����2�η�̪��Һ��Ȼ����Ba(OH)2��Һ�����ٵμ�0.2mol/LH2SO4��Һ����õ絼����ʱ��仯������ͼ��
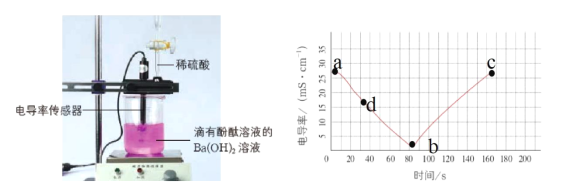
��1��0-80s�۲쵽��ʵ��������_____��
��2��д����ʵ���е����ӷ���ʽ_____��
��3������b�㣬����0.2molL-1H2SO4��Һ�����Ϊ_____mL������b��ĵ絼�ʲ��������bc�ε絼�������ӵ�ԭ��____��
��4����������ͬ��Ba(OH)2��Һ�У��ֱ�������ʵ���Ũ����ȵ�H2SO4��NaHSO4��Һ���䵼�������������Һ����仯��������ͼ��ʾ�����з�����ȷ������_____������ѡ�⣩
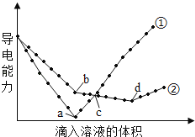
A.�ٴ����μ�H2SO4��Һ�ı仯����
B.b�㣬��Һ�д������ڵ�������Na+��OH-
C.a��d�����Ӧ����Һ��������
D.c�㣬����Һ�к�����ͬ����OH-
E.H+����������Na+ǿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵�����ʾ������ȷ���ǣ� ��
A. ��ϡ��Һ�У�H2SO4��Ba(OH)2���к���Ҫ����57.3kJ/mol
B. 2C��s��+O2��g���T2CO��g����H ��O����S��O
C. ��֪��2SO2(g) +O2(g)![]() 2SO3 (g) ����H=��98.3kJ/mol����1molSO2��0.5molO2����һ�ܱ������з�Ӧ���ų�49.15kJ������
2SO3 (g) ����H=��98.3kJ/mol����1molSO2��0.5molO2����һ�ܱ������з�Ӧ���ų�49.15kJ������
D. ��10lkPa��25��ʱ��1gH2��ȫȼ��������̬ˮ���ų�120.9kJ����������������ȼ����Ϊ241.8 kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ�ǹ�ҵ�ϴӺ�ˮ����ȡMg�����̣�����˵����ȷ����
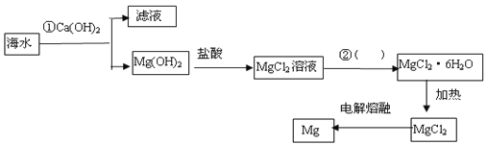
A.�ٷ�Ӧ�����ӷ���ʽ��Mg2++2OH-=Mg(OH)2��
B.�ڵ�ʵ�����������������Ũ������ȴ�ᾧ������
C.��ҵ�ϲ����õ����������þ��þ
D.MgCl26H2O����ֱ�Ӽ��ȵ���ˮ�Ȼ�þ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʵ��������ͭ��Ũ������ȡ��������̽���������ʣ���ش��������⣺
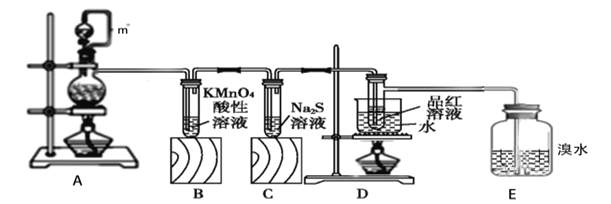
��1��װ��A��m������___��װ��A�������Ե�һ������___��
��2��װ��A�з����Ļ�ѧ��Ӧ����ʽ___���÷�Ӧ������������__________��
��3��װ��B�е�����________��֤��SO2����________��
��4��װ��C����Һ�ڿ����в��ױ��棬ʱ�䳤�˻���ֻ��ǣ�ԭ����_______���������ӷ���ʽ��ʾ��
��5��װ��D��Ŀ����̽��SO2��Ʒ�����õĿ����ԣ�д��ʵ�����������__��
��6��E�е�������___�������Ļ�ѧ��Ӧ����ʽ_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2017��3��21���ǵڶ�ʮ���������ˮ����������ˮ��Դ���������÷�ˮ��ʡˮ��Դ����ǿ��ˮ�Ļ��������ѱ�Խ��Խ���������ע����֪��ij��ɫ��ˮ�п��ܺ���H����NH4+��Fe3����Al3����Mg2����Na����NO3-��CO32-��SO42-�еļ��֣�Ϊ������ɷ֣��ֱ�ȡ��ˮ��Ʒ1L������������ʵ�飬��������й�ͼ��������ʾ��

��ش��������⣺
(1)��������3��ʵ����Է�����ˮ��һ�������ڵ���������__________��һ�����ڵ���������___________��
(2)д��ʵ���ͼ���г����ﵽ��������������ٷ����仯�η�����Ӧ�����ӷ�Ӧ����ʽ��________��
(3)����ͼ����ԭ��Һ��c(NH4+)��c(Al3��)�ı�ֵΪ______�����ó��������������______g��
(4)��ͨ��ʵ��ȷ��ԭ��ˮ��c(Na��)=0.18 mol��L-1,���ж�ԭ��ˮ��NO3-�Ƿ���ڣ�____(��������������������������ȷ����)�������ڣ� c(NO3-) = _____ mol��L-1��(�������ڻ�ȷ����˿ղ���)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com