
�ִ�п��Ʒ�ӹ���ҵ���յķ���������ZnO��FeO��Fe2O3��CuO��Al2O3�����ʣ�����ȡ����п���������£�
�й�����������ȫ������pH���±���
| ������ | Al(OH)3 | Fe(OH)3 | Fe(OH)2 | Cu(OH)2 | Zn(OH)2 |
| pH | 5.2 | 3.2 | 9.7 | 6.7 | 8.0 |
��16�֣�
��1�����ȡ����裨2�֣�
��2���ձ�����������©�� ��3�֣�
��3��ʹFe3+ת��ΪFe(OH)3������ȥ��2�֣�
Al(OH)3��Cu��Zn ��2�֣� ������Zn���۷֣�
��4��2Na2CO3+2Zn(NO3)2+H2O=4NaNO3+Zn2(OH)2CO3+CO2����2�֣�
��5������Ũ������ȴ�ᾧ�����ˣ�3�֣�
��6����©���м�����������ˮ����û������������Ȼ�˳����������Σ�2�֣�
���������������1������Ӧ��Ũ�ȡ������¶ȡ�����ѹǿ��������μӵķ�Ӧ�����������������ʹ�ú��ʵĴ�������ĥ�����衢�ȴ�ʩ�����Ǽӿ췴Ӧ���ʡ���߽����ʵij��ô�ʩ����2�����˷����Һ�������õIJ����������ձ�����ͨ©��������������3���������ǿ�����Ժ�ǿ���ԣ����������¿����е�����Ҳ�ܽ�������������Ϊ�����ӣ�����Һ�к���Zn2+��Fe3+��Cu2+��Al3+��H+��NO3������������Ϣ��֪������ҺpH����4ʱ����������ȫ��Ϊ�����������������Գ�ȥ��Һ�е������ӣ�����ҺpH����6ʱ����������ȫ��Ϊ�����������������Գ�ȥ��Һ�е������ӣ�����п��ͭ���ã�����������п���������������ӣ������ܽ�ͭ������ȫ��ԭΪ����ͭ���ȳ�ȥ���ʣ���û�����������ʣ������II����������Ҫ�ɷ�Ϊ����������ͭ��п����4�������⣬�����̼����������п��Һ��Ӧ�����ɼ�ʽ̼�ᡢ������̼�����������غ�ԭ���ɵøø��ֽⷴӦ����ʽ��2Na2CO3+2Zn(NO3)2+H2O=4NaNO3+Zn2(OH)2CO3+CO2������5���������ǿ������Σ��ܽ�����¶��½������Լ�С�������������ƶϣ�����Һ����Ũ������ȴ�ᾧ�����˵õ������ƾ��壻��6�����ݹ���֮��ϴ�ӳ�����һ��ԭ����ϴ�Ӽ�ʽ̼��пʱ������©���м�����������ˮ����û��������������Ȼ�˳����������μ��ɡ�
���㣺�����й������Ʊ��Ļ�ѧ�������̣��漰�ӿ췴Ӧ���ʵĴ�ʩ�����ˡ�������ҺpH���Ƶ�һ��ֵ��Ŀ�ġ������ɷ֡��ؼ�����Ļ�ѧ��Ӧ����ʽ����ȡ����ķ�����������ϴ�ӵȡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧС���������������������װ�ã���ͼ�����Ի������Ʊ�����ϩ��
��֪��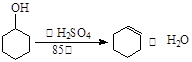
| | �ܶ� ��g/cm3�� | �۵� ���棩 | �е� ���棩 | �ܽ��� |
| ������ | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | ��103 | 83 | ������ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�Ϸ���ijУ��ѧ��ȤС��Ϊ̽������Ũ���ᷴӦ�����������ͼ��ʾװ�ý���ʵ�顣
(1)ʵ������У��۲쵽B�е�ʵ�������� ��
һ��ʱ��۲쵽��C��������������ð���������ݵijɷ��� ��
(2)�á��ɳ鶯����˿�����桰ֱ��Ͷ����Ƭ�����ŵ��� ����Ӧ��������Ҫ�������Ϳ�ʹװ���в���������ȫ�����գ�Ӧ����ȡ�IJ����� ��
(3)��Ӧһ��ʱ������Ƕ�A����Һ�Ľ��������ӽ�����̽����
��������裺
����1����Һ��ֻ����Fe2+��
����2�� ��
����3����Һ�д���Fe2+��Fe3+��
���������ʵ����֤��������1��д��ʵ�������ʵ�������ۡ�
��ѡ�Լ���KMnO4��Һ��NaOH��Һ������һKI��Һ��KSCN��Һ
| ʵ��������� | ʵ������ | ���� |
| | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�{����������a-Fe2O3)���ִ����ӹ�ҵ��Ҫ���ϡ�ʵ���������������� (Fe2O3��FeO��SiO2��)Ϊԭ���Ʊ��{���������IJ������¡���ش��й����⣺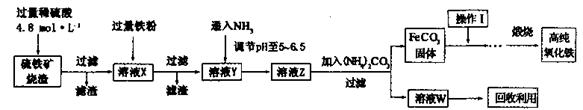
��1�����˲����еIJ�������Ϊ________��
��2��ʵ������240mL4.8mol ? L��1��������Һ������18.4 mol ? L��1��Ũ����������ƣ�����Ҫ������Ҫ����Ϊ________��
��3����ҺX������Ӧ�����ӷ���ʽΪ________��
��4��������μ�����ҺZ�е�������________��
��5������I��������________���о�W��һ����;________��
��6�� ijʵ��С����Ƶİ����Ʊ�ʵ������Ϊ:����װ�á��������ռ���β����������ӿڵ�����˳����_______________��
��7��������İ����ֱ�ͨ��ˮ�л�������,���õ�25��0.1 mol.L-1��NH3 ? H2O��Һ��NH4Cl��Һ���������ʵ�飬�Ƚ�NH3 ? H2O�ĵ���̶Ⱥ�NH4Cl��ˮ��̶ȴ�С��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijʵ��С�����ͨп�̷ϸɵ���ڵĺ�ɫ�������̽����������·�����
��֪��I����ͨп�̵�صĺ�ɫ������Ҫ�ɷ�ΪMnO2��NH4Cl��ZnCl2�����ʡ�
II��������пΪ��ɫ��ĩ��������ˮ�������ᡢǿ����Һ�Ͱ�ˮ��
��ش��������⣺
��1���ڲ�����������___________��
��2��ijͬѧ������ҺA�ijɷֺ���NH4Cl��ZnCl2���������һ��ʵ�鷽������֤�������ȷ��Ҫ���ڴ���ϰ��±���ʽд��ʵ�������Ԥ������ͽ��ۡ�
��ѡ�Լ�������ˮ��2moL��L��1 HCI ��2 moL��L��1 HNO3 ��2 moL��L��1 NH3��H2O��6 moL��L��1 NaOH��0.1 moL��L��1 KSCN��0.1 moL��L��1 BaCl2��0.1 moL��L��1 AgNO3����ɫʯ����Һ����ɫʯ����ֽ
| ʵ����� | Ԥ������ | ���� |
| ����1����ȡ������ҺA��װa��b��c��֧�Թܣ���a�Թܣ�__ __________________________ | �а�ɫ�������� | ˵����ҺA����Cl�� |
| ����2����b�Թܣ�__________ __________________________ | ______________________ | _______________________ |
| ����3����c�Թܣ�__________ __________________________ | �Ȳ���_______________, ��____________________ | ˵����ҺA����Zn2+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������������;�㷺����ҵ�ϳ��ú�����Al2O3�ķ�����FeO��V2O5�����۷���ȡV2O5����Ҫ�����������£�
��֪���ٱ���ʱ�ɷ�����Ӧ��V2O5+Al2O3+2Na2CO3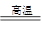 2NaVO3+2NaAlO2+2CO2��
2NaVO3+2NaAlO2+2CO2��
�ڳ��������ʵ��ܽ�ȣ�NaVO3��21.2 g /100gˮ��HVO3��0.008 g/100gˮ
��1����������B������Ҫ�ɷ��� ����д��ѧʽ��
��2�������У���ֱ����H2SO4���ݡ�����A����ȡHVO3��ԭ���� ��
��3���������١����� ��ϴ�ӡ������ϴ�ӣ����Ʒ�п��ܺ��еĽ����������� �� ������װ�ã����ּг�����ʡȥ��������ʵ���ҽ��С������ڡ����� ��������ţ�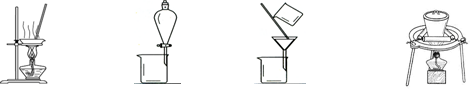
A B C D
��4��NaVO3����ԭ�͵�����������V2O5����NaOH��Һ����ȡ����Ӧ�����ӷ���ʽΪ ��
��5��V2O5�������ڽ��������ɷ����������м���CaO�ɽ��ܼ��š��йط�Ӧ���£�
2V2O5(l)+ 5Si(s)+ 5CaO(s)=" 4V(s)+" 5CaSiO3(s) ��H1 =" ��2096.7" kJ/mol
��֪��CaSiO3(s)=" CaO(s)+" SiO2(s) ��H2 =" +92.5" kJ/mol
��2V2O5(l)+ 5Si(s)=" 4V(s)+" 5SiO2(s) ��H3 = ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���ͷ���һ����������Ʒ����ʳƷ�Ļ�ѧ���ɼ�����С�մ���(̼�����)�������е�����������ɡ�ij�о���ѧϰС��Ϊ̽����ͬƷ�Ƶķ��ͷ۵Ļ�ѧ�ɷ֣���������ʵ�顣
��������衿
��1������1����С�մ�ͳ�����ɣ�
����2����С�մ��������ɣ�
����3����________________��ɡ�
�����������̡�
Ϊ̽��ijƷ�Ƶķ��ͷ۵Ļ�ѧ�ɷ֣�ijͬѧ�������ʵ�飬�õ���������
��2���÷��ͷ۵ijɷ�Ϊ________ (�ѧʽ����
��3����һƷ�Ƶķ��ͷ۵Ļ�ѧ��ɿ���Ϊ����2������������ʵ����֤��д��ʵ�鲽�衢Ԥ������ͽ��ۡ�
| ʵ�鲽�� |  Ԥ��������� Ԥ��������� |
| 1��ȡ������Ʒ����ϡ�������Һ�ֳ����� | |
| 2�� _______________________________________ | |
| 3�� ________________________________________ | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij����С���һ����̼��ԭ��������ʵ�������IJ������Ũ�����Ȥ����ͨ��ʵ����̽����ɷ֡�
��ʵ��װ�ã�
��һ����̼��ԭ��������ʵ��װ��
��װ��B�з��������ӷ���ʽ��
װ��B��������
��ʵ����������A�еķ�ĩ�ɺ�ɫ��Ϊ��ɫʱ��ֹͣ���ȣ�����ͨһ����̼����ȴ�����£�ֹͣͨ����ͬʱ�۲쵽�����ʯ��ˮ����ǡ�
��ʵ����ۣ�
����Ϊ����������ʵ����������жϳ����ɵĺ�ɫ����Ϊ��������
����Ϊ����������ʵ����������֤�����ɵĺ�ɫ����Ϊ����������������һ��ʵ�飺�ô����������ɵĺ�ɫ���壬�����к�ɫ���屻�������������ǵó����ɵĺ�ɫ����Ϊ�������Ľ��ۡ�
����ͨ���÷�Ӧ��������϶����ǽ��������жϲ�ͨ��ʵ�����������ԣ�
��1����һ�������£�һ����̼���������ڼ��������£��ɷ������·�Ӧ
3Fe2O3+CO 2Fe3O4+CO2
2Fe3O4+CO2
Fe3O4+4CO 4Fe+4CO2
4Fe+4CO2
��2��������������Fe3O4��Ϊ��ɫ���壬��ǿ���ԣ��ܹ�������������
�ס���ͬѧ�Ľ��ۣ� ��Դ����۵������ǣ�
����ʵ��̽��
�Է�Ӧ�����ɷ�������裺
����1����Ӧ�������ֻ��Fe��
����2����Ӧ�������ֻ��Fe3O4��
����3����Ӧ�������_______________________
Ϊȷ��ʵ�������IJ���ijɷ֣���ͬѧ�������ʵ�飬����������ѡ�Լ���������������ɸ�̽�����̣�������д�ڴ����Ӧλ�á�
��ѡ�Լ��������� 1mol/LCuSO4 ��0.01mol/L KSCN��Һ��1mol/L���ᡢ0.01mol/L��ˮ���Թܡ�����������ͷ�ιܡ�
| ʵ����� | Ԥ������ͽ��� |
| ����һ��ȡӲ�ʲ������й�����������ֱ���A��B�Թ��У���������1mol/LCuSO4��Һ�������ܽ⡣ | ��1����A�Թ��к�ɫ���岻�ܽ⣬����û�й۲쵽�����������ɫ����Ϊ ��2����B�Թ����к�ɫ������������˵����ɫ�����к���Fe�� |
| ����������Թ�B����Һ���ˣ������ù���ϴ�Ӹɾ�������1mol/L����������ηֱ��������0.01mol/L��ˮ������0.01mol/L KSCN��Һ | ��1������Һ�����ɫ���� ��2������Һ���ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
ʵ������ijЩ�������ȡ���ռ���β������װ����ͼ��ʾ(ʡ�Լгֺ;���װ��)�����ô�װ�úͱ����ṩ������������ʵ�飬�������ѡ���� (����)��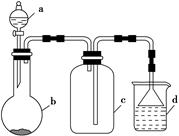
| ѡ�� | a�е����� | b�е����� | c���ռ������� | d�е����� |
| A | Ũ���� | MnO2 | Cl2 | NaOH��Һ |
| B | Ũ���� | Na2SO3 | SO2 | NaOH��Һ |
| C | Ũ��ˮ | CaO | NH3 | H2O |
| D | ϡ���� | Cu | NO2 | H2O |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com