

| ������ | Al��OH��3 | Fe��OH��3 | Fe��OH��2 | Cu��OH��2 |
| pH | 5.2 | 3.2 | 9.7 | 6.7 |
| H2 |
| 550�� |
| c(H+)2 |
| c(Cu2+) |
| c(H+)2 |
| c(Cu2+) |
| Kw2 |
| Ksp |
| (1��10-14)2 |
| 2.0��10-20 |
| 2000��60% |
| 160 |
| 2000��36% |
| 72 |
| 34g |
| 17g/mol |
| H2 |
| 550�� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
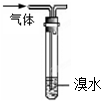
| A���ø���pH��ֽ�ⶨij������ˮ��pH |
| B���þƾ���ȡ��ˮ�еĵ� |
| C������ͼװ���ܳ�ȥ�����л��е���ϩ |
| D����25mL��ʽ�ζ�����ȡ20.00mL KMnO4��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��100mL 1mol/L MgCl2��Һ |
| B��200mL 0.25mol/L AlCl3��Һ |
| C��100ml 1mol/L NaCl��Һ |
| D��200ml 0.5mol/L HCl��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| 3 |
| 2 |
| T/K | 303 | 313 | 323��] |
| NH3������/��10-6mol�� | 4.8 | 5.9 | 6.0 |
| 3 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
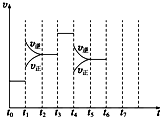 ��һ�ܱ������з�����ӦN2+3H2?2NH3��H��0���ﵽƽ���ֻ�ı�ijһ������ʱ����Ӧ�����뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ��
��һ�ܱ������з�����ӦN2+3H2?2NH3��H��0���ﵽƽ���ֻ�ı�ijһ������ʱ����Ӧ�����뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | CH3COONa | NaHCO3 | Na2CO3 | NaClO | NaCN | C6H5ONa |
| PH | 8.1 | 8.4 | 11.6 | 10.3 | 11.1 | 11.3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com