
��12�֣���ͼΪ100mL 2 mol /L ��AlCl3��Һ�м���NaOH��Һ����Al(OH)3��ɫ������������NaOH�����ʵ���֮��Ĺ�ϵ���ߡ��Իش�

(1) A��ʱ�Ѳμӷ�Ӧ��AlCl3�� NaOH�����ʵ���֮��Ϊ1�� ��
(2) AB����������ʾ�ķ�Ӧ�����ӷ���ʽ��___________________��
(3) B����Һ�д��ڵ����ʵ���Ũ������������ �������ӷ��ţ�����B�����ɵ���Һ��ͨ�������̼���ɼ����������� ��
��4�����ڸ�AlCl3��Һ������7.8�˳����������ĵ��������Ƶ����ʵ���Ϊ mol�� mol��
��12�֣�ÿ��2�֣� (1) 3��
(2) Al(OH)3��OH����AlO2����2H2O��
(3) Na+ �����ɰ�ɫ������
��4��0.3mol��0.7mol
��������A����������������ʱ���з�Ӧ�պ���ɣ�
AlCl3��3NaOH=Al(OH)3����3NaCl, AB����������
ʾ�ķ�Ӧ�dz����պ��ܽ⣺Al(OH)3��NaOH=NaAlO2��2H2O,���ӷ�ӦΪ
Al(OH)3��OH����AlO2����2H2O�����B����Һ�д��ڵ�������NaCl��NaAlO2Ũ������������Na+����B�����ɵ���Һ��ͨ�������̼�������·�Ӧ��NaAlO2��CO2��2H2O=Al(OH)3����NaHCO3,�а�ɫ����������
�ȵ������������٣�AlCl3��3NaOH=Al(OH)3����3NaCl
3mol 78g
n 7.8g n=0.3mol
�������������࣬AlCl3ȫ����Ӧ����������������ܽ�һ����
AlCl3��3NaOH=Al(OH)3����3NaCl
1mol 3mol 78g
0.2mol n m n=0.6mol m=15.6g
Al(OH)3��NaOH=NaAlO2��2H2O
78g 1mol
15.6g-7.8g n1 n1 =0.1mol�������ĵ��������Ƶ����ʵ���Ϊ0.6mol+0.1mol=0.7mol��


 ����ѧУ�ֲ����ܲ�ϵ�д�
����ѧУ�ֲ����ܲ�ϵ�д� �ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
�ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ����������Һ ����ʽ��NaOH ��Է���������40 �ܶȣ�1.2g?cm-3 ����������20%��1����NaOH��Һ�����ʵ���Ũ��Ϊ 6 6 mol/L����2������Ҫ���Ƹ�Ũ�ȵ�NaOH��Һ100ml������� 24.0 24.0 g�����������ƣ���Һ���Ƶ�����Ļ����������£� ��3��������ʵ�鲽��A��F��ʵ������Ⱥ�������� CBDFAE CBDFAE ����4������ʵ�鲽��A��B��E��F���õ�����������Ϊ 100ml����ƿ 100ml����ƿ ����5�����в�����NaOH��Һ�����ʵ���Ũ���к�Ӱ�죨�ƫ�ߡ�����ƫ�͡�����Ӱ�족���� ��ҡ�Ⱥ���Һ����ڿ̶����ټ�ˮ ƫ�� ƫ�� ��������ƿ��ԭ����������ˮ ��Ӱ�� ��Ӱ�� ���۶���ʱ���ӹ۲�Һ�� ƫ�� ƫ�� ��
�鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ����� 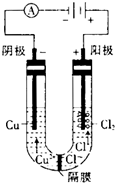 ��ͼΪϸ��ұͭ�ͻ�ұͭ����Ҫ���̣� ��ͼΪϸ��ұͭ�ͻ�ұͭ����Ҫ���̣�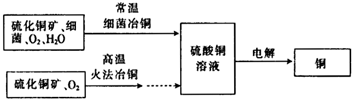 ��1����д���ö��Ե缫�������ͭ��Һ�Ļ�ѧ����ʽ�� �������һ��ʱ������0.1mol��������ͭ��ʹ���Һ�ָ������ǰ��Ũ�ȣ���·��ת�Ƶ������ʵ��� ��2��ϸ��ұ���ֳ���������ǽ���ʪ��ұ��ҵ�ϵ�һ���¹��գ�ϸ��ұͭ���ұͭ��ȣ��ŵ�Ϊ ��3���ö��Ե缫�ֱ���Ũ���Ȼ�ͭ��Һ������ͭ��Һ�����Ũ���Ȼ�ͭ��Һʱ���������н���ͭ���ɣ�ͬʱ��������������غ�ɫ��Һ�����������ͭ��Һʱ��û���غ�ɫ��Һ���ɣ������ǹ����غ�ɫ��Һ�ɷֵ�̽���� ����ͬѧ��Ϊ�������������ֵ��غ�ɫ��Һ��������Ӧ�Ľ��������Ϊ���IJ²��Ƿ���ȷ�� ����1�� һ����л�ϼ�̬��ָ��������ͬһԪ�ش������ֲ�ͬ�Ļ��ϼۣ���Fe3O4�е�FeԪ���������ʵ���ɫ�ȵ�һ��̬�����ʵ���ɫҪ� ����2�� CuCl����ˮ��������Ũ���ᣮ �ڲ��룺�غ�ɫ��Һ�п��ܺ��е������� ����֤���룺���ʵ�鷽���������غ�ɫ��Һ����ȡ���� ����֪���ǰ��U�ι��м�����100mL 0.5mol?L-1 CuCl2��Һ������cʱ��·��һ��ת����0.03mol���ӣ�����������0.64gͭ�����γɵĵͼ������ӵ����ʵ���Ϊ �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ� ��12�֣���ͼΪ100mL 2mol /L ��AlCl3��Һ�м���NaOH��Һ����Al(OH)3��ɫ������������NaOH�����ʵ���֮��Ĺ�ϵ���ߡ��Իش� (1) A��ʱ�Ѳμӷ�Ӧ��AlCl3�� NaOH�����ʵ���֮��Ϊ1�� �� (2) AB����������ʾ�ķ�Ӧ�����ӷ���ʽ��___________________�� (3) B����Һ�д��ڵ����ʵ���Ũ������������ �������ӷ��ţ�����B�����ɵ���Һ��ͨ�������̼���ɼ����������� �� ��4�����ڸ�AlCl3��Һ������7.8�˳����������ĵ��������Ƶ����ʵ���Ϊ mol�� mol��
�鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ��2011��2012ѧ�꽭��ʡ������ѧ��һ��ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������� ��12�֣���ͼΪ100mL 2 mol /L ��AlCl3��Һ�м���NaOH��Һ����Al(OH)3��ɫ������������NaOH�����ʵ���֮��Ĺ�ϵ���ߡ��Իش� �鿴�𰸺ͽ���>> ͬ����ϰ��� ����ѧУ��ѡ - ��ϰ���б� - �����б� ����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר�� Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com��Ȩ��������վ�������£�ͼƬ��Դ�����磬����Ȩ����Ȩ��ԭ�������У�ת�������ַ���Ȩ��������Ȩ����������������֪�����ǽ����촦������ϵqq��3310059649�� ICP�������: ��ICP��07509807��-10 ����������42018502000812�� |