
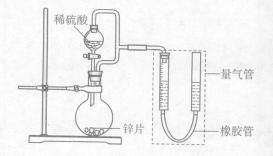
 ��
��| ��� | V��H2SO4��/mL | C(H2SO4)/mol��L-1 | t/s |
| I | 40 | 1 | t1 |
| II | 40 | 4 | t2 |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
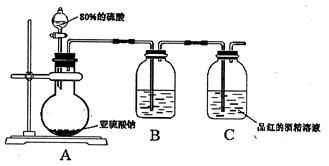
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����



| ѡ�õ�����������ĸ�� | ������Լ� | ���� |
| | | ��Ӧ�����������壩 |
| C | | |
| | ����ͭ | ʹ����������ͭ��Ӧ |
| | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ѡ�� | ���缫 | ����� | ������Ӧ | ������Ӧ |
| A | ���� | NaOH | Al-3e-��Al3+ | 2H2O+2e-��2OH-+H2�� |
| B | ���� | ϡ���� | 2Al-6e-=2Al3+ | 6H++6e-��3H2�� |
| C | ���� | Ũ���� | Cu-2e--��Cu2+ | 2NO3-+4H+-4e-��2NO2��+2H2O |
| D | ���� | ϡ���� | Cu-2e-��Cu2+ | 2NO3-+8H+��2NO��+4H2O+6e- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
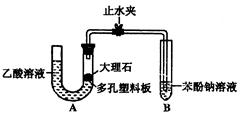
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 �����ӵ�±ˮΪ��Ҫԭ���Ʊ���ˮ
�����ӵ�±ˮΪ��Ҫԭ���Ʊ���ˮ ��
�� ���������£�
���������£�
 ��
�� ���ɱ������ݿ�֪�������Ͽ�ѡ��pH���Χ�� ���ữ��ҺZʱ��ʹ�õ��Լ�Ϊ ��
���ɱ������ݿ�֪�������Ͽ�ѡ��pH���Χ�� ���ữ��ҺZʱ��ʹ�õ��Լ�Ϊ ��
 ���壬����װ���к������� ��
���壬����װ���к������� ��
 �ĵ��볣��
�ĵ��볣�� ��
�� ��
�� �ĵ�
�ĵ� �볣��
�볣�� ��
�� ��ijͬѧ���ʵ����֤
��ijͬѧ���ʵ����֤ ����ǿ��
����ǿ�� ����
����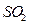 ��
�� ����ֱ�ͨ��ˮ�������ͣ���������ȼƲ�����Һ��
����ֱ�ͨ��ˮ�������ͣ���������ȼƲ�����Һ�� ����ǰ�ߵ�
����ǰ�ߵ� С�ں��ߣ���
С�ں��ߣ��� ����ǿ��
����ǿ�� ����ʵ����Ʋ���ȷ���������� ��
����ʵ����Ʋ���ȷ���������� �� ����ǿ��
����ǿ�� ����Ҫ˵��ʵ�鲽�衢����ͽ��ۣ��� ����������ѡ��
����Ҫ˵��ʵ�鲽�衢����ͽ��ۣ��� ����������ѡ�� ��
��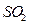 ��
�� ��
�� ��
�� ��
�� ������ˮ������ʯ��ˮ������
������ˮ������ʯ��ˮ������ ��Һ��Ʒ����Һ��
��Һ��Ʒ����Һ�� ��ֽ��
��ֽ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ��������� | ʵ�鲽�� | ʵ������ | ʵ����� |
| ����2��Сֽ���е����� �ܷ������������� | ȡ����Сֽ���й�������ձ��У���������ˮ���������ڡ� | | ������ ����� |
| ����3���Ҳ�����ʺ�����ʿ�����̼��ƣ��������֤�ҵIJ��룿 | | | �ø������Ʒ����̼��� |
 .
.�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com