
����Ŀ��ijС��ȡһ��������FeSO4���壬������ͼװ�ý���ʵ�顣


��֪��SO2�۵㣭72�棬�е㣭10�棻SO3�۵�16.8�棬�е�44.8 �档
(1)ʵ��۷�Ӧ�����ӷ���ʽ��_________________________��
(2)�ֽ���̳�����ʹľ����ȼ�������⣬����A�й�����ɫ�仯�Ʋ⣬��һ����________���壬������________________________________��
(3)ʵ��ܷ�Ӧ�����ӷ���ʽ��__________________________��
(4)ijͬѧ����B�е�������ΪFeSO4�ֽ�һ����SO3���ɡ�����Ϊ�Ƿ���ȷ����˵��ԭ��____________(�ñ�Ҫ�����ֺͻ�ѧ����ʽ����)��
���𰸡�Fe2O3��6H��=2Fe3����3H2O SO2 ��Fe2O3���ɣ���FeSO4��ֻ�У�6��S��������(�ܱ���ԭ)�����һ����SO2���� 2Fe3����SO2��2H2O=2Fe2����SO42-��4H�� ����ȷ����Ϊ�ֽⷴӦ��O2��SO2���ɣ�ˮ��Һ�з�����Ӧ��2SO2��O2��2H2O=2H2SO4�������۷ֽⷴӦ�Ƿ���SO3���ɣ������д�����
��������
(1)A�й����Ϊ����ɫΪ��������Ȼ������ᣬ�������������ᷴӦ���ݴ���д���ӷ�Ӧ����ʽ��
(2)�ֽ���̳�����ʹľ����ȼ������Ϊ������A�й����Ϊ����ɫΪ����������Ϊ��Fe2O3���ɣ���FeSO4��ֻ��+6��SԪ���������ԣ��ܱ���ԭ�����һ����SO2���ɣ�
(3)AΪ�������������������������ӣ�����������Һ����D�Թ��У���Һ��Ϊdz��ɫ˵���ж��������ɣ��������������ӺͶ�����������������ԭ��Ӧ���ݴ˽��
(4)FeSO4�ֽⲻһ����SO3���ɣ�B�е��������а�ɫ�������ɣ���������ˮ��Һ�з�����Ӧ��2SO2+O2+2H2O=2H2SO4��H2SO4+BaCl2=BaSO4��+2HCl�������۷ֽⷴӦ�Ƿ���SO3���ɣ������д����ݴ˽��
(1)���ݢ�ľ����ȼ˵�����������ɣ�����A�й����Ϊ����ɫ��˵��A�й����Ϊ����ɫΪ��������B���а�ɫ����Ϊ���ᱵ������Ϊ���������ڵ����������¸����������ȷֽ�������������������������ʵ��۷�Ӧ�����ӷ���ʽ��Fe2O3+6H+=2Fe3++3H2O��
(2)����������ԭ��Ӧ���ص㣬Ԫ�ػ��ϼ����ߵļ�������Ԫ�ػ��ϼ۽��͵ļ������ֽ���̳�����ʹľ����ȼ������Ϊ����˵����Ԫ�صĻ��ϼ������ߣ�A�й����Ϊ����ɫΪ����������Ϊ��Fe2O3���ɣ����Ļ��ϼ�Ҳ�����ߣ���FeSO4��ֻ��+6��SԪ���������ԣ��ܱ���ԭ�����һ����SO2���ɣ�
(3)ȡA�й��壬������Fe2O3+6HCl=2FeCl3+3H2O������������Һ����D�Թ��У���Һ��Ϊdz��ɫ˵���ж��������ɣ�˵�����������ӱ���ԭ��D�Թ�������ɫҺ��Ϊ�����������ķ�ӦΪ2Fe3++SO2+2H2O=2Fe2++SO42-+4H+��
(4)����ȷ����Ϊ���������ڵ����������¸����������ȷֽ�һ����������������������һ������������B�е��������а�ɫ�������ɣ���ɫ����Ϊ���ᱵ���ó���������2SO2+O2+2H2O=2H2SO4��H2SO4+BaCl2=BaSO4��+2HCl�������۷ֽⷴӦ�Ƿ���SO3���ɣ������д������ܷ�ӦΪ2SO2+O2+2H2O+2BaCl2=2BaSO4��+4HCl��


 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ס��ҡ�������������������ڵ����ʼ�������ͼ��ʾ����������������ݻ���ȣ��¶���ͬ����Ӧ�мס������ݻ����䣬���е�ѹǿ���䣬��һ���¶��·�Ӧ�ﵽƽ�⡣����˵����ȷ����
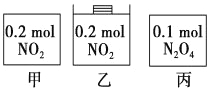
A. ƽ��ʱ��������c��NO2���Ĵ�С˳��Ϊ��>��>��
B. ƽ��ʱN2O4�İٷֺ�������>�ף���
C. ƽ��ʱ����NO2�����N2O4��ת������ͬ
D. ƽ��ʱ������ƽ����Է�����������>��>��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������β���к���CO��NOx���ж����壬��������װβ������װ�ã���ʹ�ж��������Ӧת��Ϊ�����塣
��1����֪ 4CO(g)��2NO2(g)![]() 4CO2(g)��N2(g) ��H����1200 kJ��mol1
4CO2(g)��N2(g) ��H����1200 kJ��mol1
�ٸ÷�Ӧ��________________���������¡����»��κ��¶����������Է����С�
�ڶ��ڸ÷�Ӧ���ı�ijһ��Ӧ�������¶�T1>T2��������ͼ����ȷ����_______(�����)��
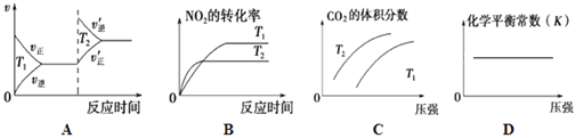
��ijʵ��С��ģ�������������̣�һ���¶��£���2L�ĺ����ܱ������У���ʼʱ���ռס������ַ�ʽ����Ͷ�ϣ�����һ��ʱ���ﵽƽ��״̬����ü���CO��ת����Ϊ50%����÷�Ӧ��ƽ�ⳣ��Ϊ__________�����ַ�ʽ��ƽ��ʱ��N2�������������______�ң� ����>��=��<��ȷ��������ͬ����NO2��Ũ�ȣ���______�ҡ�

��2����������β���е�̼��(C)��NOx��ͨ��ij���ܴ�������������ͬ�¶��£���ģ��β�����ɷ����±���ʾ������ͬ������ͨ���ô���������в���(CO2��N2��N2O)��NO��������ݽ����ͼ��ʾ��
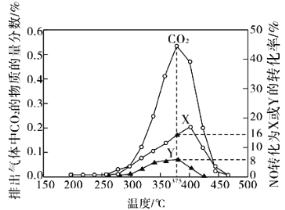
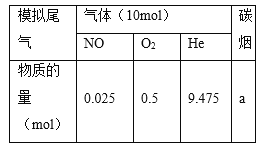
��375��ʱ������ų��������к�0.45 mol O2��0.0525 mol CO2����Y�Ļ�ѧʽΪ________��
��ʵ������в���NOģ��NOx����������NO2��ԭ����__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��п��п�Ļ�����Ӧ�ù㷺�����磬�ⶨͭ�Ͻ��е�Ǧ��пʱҪ����п�����ӵ����з�Ӧ��[Zn(CN)4]2-��4HCHO��4H2O=Zn2+��4HOCH2CN��4OH��
�ش��������⣺
(1)��̬Zn2+�ĵ����Ų�ʽΪ_____________����̬ Cԭ�Ӻ������ռ��_____����ͬԭ�ӹ����
(2)C��N��O����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ___________��HOCH2CN�����к��еĦҼ���м���Ŀ֮��Ϊ_________��
(3)HCHO������̼ԭ�ӹ�����ӻ�������________������������HCHO��ˮ��Һ��HCHO������ˮ���ܵ���Ҫԭ����_________________________��
(4)[Zn(CN)4]2-��Zn2+��CN��֮��Ļ�ѧ����Ϊ_________���ṩ�µ��ӶԵijɼ�ԭ����________��
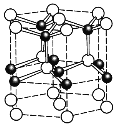
(5)ZnO��һ�����ȶ��ľ���ṹ��ͼ��ʾ��������Zn2+����λ��Ϊ______���������ױ߳�Ϊacm����Ϊbcm���谢���ӵ�������ֵΪNA����ZnO���ܶ�Ϊ_______ g/cm3���г��������ʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ����ģ��ϳɰ��Ͱ����������������£�

��֪ʵ���ҿ��ñ�����������(NaNO2)��Һ�뱥���Ȼ����Һ�����Ⱥ�Ӧ��ȡ������
(1)����ͼ��ѡ����ȡ����ĺ���װ�ã�����________������________��
(2)����������ͨ����װ�ã���װ�õ����ó��˽��������⣬����________��________��

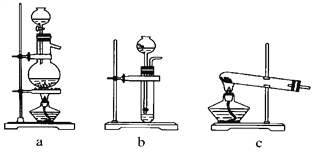
(3)���ϳ�����������ȴ����������ͨ����װ�õ�ˮ�����հ���________(���ᡱ���ᡱ)����������ԭ����_________________��
(4)����װ������һ��ʱ�䰱����ͨ�������ͬʱ�������ȵIJ�˿������װ�õ���ƿ�ڣ���ʹ��˿���ֺ��ȵ�ԭ����________����ƿ�л��ɹ۲쵽��������____________________________��
(5)д����װ���а������Ļ�ѧ����ʽ��_____________________________________________��
(6)��Ӧ��������ƿ�ڵ���Һ����H����OH����______��________���ӡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£����и���������ָ����Һ���ܴ����������
A. ![]() ����Һ�У�K+��Na+��CO32-��NO3-
����Һ�У�K+��Na+��CO32-��NO3-
B.����������Һ�У�Fe3+��Mg2+��SCN-��Cl-
C.c(Fe2+)=1 mol/L����Һ�У� Na+��NH4+��AlO2-��SO42-
D.��ʹ���ȱ�����Һ�У� K+��NH4+��SO42-��HCO3-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������йػ�ѧ��������ᷢչ���̵�˵���У�����ȷ����![]()
![]()
A.�����ٵ���ԭ�������Ͱ����ӵ���������ѧ˵���Ի�ѧ�ķ�չ���˼�����ƶ�����
B.�Ž��з�Ԫ�ذ�ԭ��������С�����˳���������У��Ƴ��˵�һ��Ԫ�����ڱ�
C.���������֮������ν������ĸ�춦��������ͭ��Ʒ
D.![]() ���ݸ�Ŀ
���ݸ�Ŀ![]() ����������������н��֮�ң������Ի���֭��ȡ��������еļ���
����������������н��֮�ң������Ի���֭��ȡ��������еļ���![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ӦA��g��+3B��g��2C��g��+2D��g�������ֲ�ͬ����µķ�Ӧ�������£����б�ʾ��Ӧ����������
A. v��A��=0.15molL-1min-1 B. v��B��=0.01molL-1s-1
C. v��C��=0.40molL-1min-1 D. v��D��=0.0075molL-1s-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������üױ�Ϊԭ�Ϻϳɣ���֪�����ϵ������ɱ���ԭΪ������![]() +3Fe+6HCl��
+3Fe+6HCl��![]() +3FeCl2+2H2O�����ﱽ����ԭ��ǿ���ױ����������ɼױ��ϳɶ���������IJ���������ǣ� ��
+3FeCl2+2H2O�����ﱽ����ԭ��ǿ���ױ����������ɼױ��ϳɶ���������IJ���������ǣ� ��
��֪����CH3Ϊ�ڡ���λȡ����λ��������COOHΪ��λȡ����λ��
A.�ױ�![]() X
X![]() Y
Y![]() ����������
����������
B.�ױ�![]() X
X![]() Y
Y![]() ����������
����������
C.�ױ�![]() X
X![]() Y
Y![]() ����������
����������
D.�ױ�![]() X
X![]() Y
Y![]() ����������
����������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com