
����Ŀ���±���Ԫ�����ڱ���һ���֡�����Ԫ���ڱ��е�λ�ã����û�ѧ����ش���������:
�� ���� | IA | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
1 | �� | |||||||
2 | �� | �� | �� | |||||
3 | �� | �� | �� | �� | ||||
4 | �� | |||||||
5 | �� |
��1�������ۺ�����Ļ�ѧʽΪ_________��
��2���ٺ͢�����Ԫ�ص�ԭ�Ӱ�1:1��ɵij���������ĽṹʽΪ_________��
��3���ۢܢߢ�ļ�����뾶�ɴ�С��˳��Ϊ_________��(�����ӷ��ű�ʾ)
��4���ڢܵ�����������ˮ����֮�䷢����Ӧ�����跽��ʽ_________��
��5���õ���ʽ��ʾ�ۺ͢���ɵĻ�������γɹ���_________��
��6�������к��Т�Ԫ�أ��������к��и�Ԫ�صļ������ӡ��������ữ�£�����˫��ˮ��������Ϊ���ʡ�д���÷�Ӧ�����ӷ���ʽ_________��
���𰸡� HBrO4 H-O-O-H Cl->O2->Mg2+>Al3+ Al(OH)3+OH-=AlO2-+2H2O 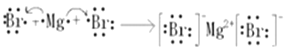 H2O2+2I-+2H+=2H2O+I2��
H2O2+2I-+2H+=2H2O+I2��
����������Ԫ�������ڱ���λ�ã���֪��ΪH����ΪNa����ΪMg����ΪAl����ΪC����ΪN����ΪO����ΪCl����ΪBr����ΪI��
��1����ΪBr��Br���������Ϊ+7��������ۺ�����ΪHBrO4��
�ʴ�Ϊ�� HClO4��
��2���ٺ͢�����Ԫ�ص�ԭ�Ӱ�1:1��ɵij���������ΪH2O2����ṹʽΪH-O-O-H��
�ʴ�Ϊ��H-O-O-H��
��3���ۢܢߢ�ļ����ӷֱ�Ϊ��Mg2+��Al3+��O2-��Cl-��ǰ�������ӵĺ�������Ų���ͬ����������İ뾶����С��Cl-���������Ӳ㣬�ʰ뾶���
�ʴ�Ϊ��Cl->O2->Mg2+>Al3+��
��4���ڢܵ�����������ˮ����ֱ�ΪNaOH��Al(OH)3,���߷�Ӧ�����ӷ���ʽΪ��
Al(OH)3+OH-=AlO2-+2H2O��
�ʴ�Ϊ��Al(OH)3+OH-=AlO2-+2H2O
��5���ۺ͢��γɵĻ�����ΪMgBr2�����ӻ�������õ���ʽ��ʾ���γɹ������£�![]() ��
��
��6�������к���IԪ�أ��������к���I-���������ữ�Ļ����£���˫��ˮ�ɽ�������Ϊ�ⵥ�ʡ��ʴ�Ϊ��H2O2+2I-+2H+=2H2O+I2��


 �����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ȼ�ѧ����ʽ��C(s)+H2O(g)![]() CO(g)+H2(g) ��H=+131.3 kJ/mol
CO(g)+H2(g) ��H=+131.3 kJ/mol
H(g)+ H(g)![]() H2(g) ��H=435.7 kJ/mol
H2(g) ��H=435.7 kJ/mol
����˵����ȷ����
A����ԭ�ӵ�����������ӵ�������
B��2����ԭ�ӽ������1������ӣ��ų�435.7 kJ������
C��1 mol��̬̼��1 molˮ������Ӧ����һ����̼�����������������131.3 kJ
D����̬̼��Һ̬ˮ��Ӧ����һ����̼�������������131.3 kJ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й�˵������ȷ����
A����ӦNH3(g)��HCl(g)![]() NH4Cl(s)�������¿��Է����У���÷�Ӧ�Ħ�H��0
NH4Cl(s)�������¿��Է����У���÷�Ӧ�Ħ�H��0
B��CaCO3(s)![]() CaO(s)��CO2(g)�����²����Է����У�˵���÷�Ӧ�Ħ�H��0
CaO(s)��CO2(g)�����²����Է����У�˵���÷�Ӧ�Ħ�H��0
C��һ���¶��£���ӦMgCl2(l)![]() Mg(l)��Cl2(g)�Ħ�H>0����S>0
Mg(l)��Cl2(g)�Ħ�H>0����S>0
D�������£���ӦC(s)��CO2(g)![]() 2CO(g)�����Է����У���÷�Ӧ�Ħ�H>0
2CO(g)�����Է����У���÷�Ӧ�Ħ�H>0
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ȥþ���л��е�������������ѡ�õ��Լ��� ��������
A. ϡ���� B. ����������Һ C. Ũ��ˮ D. ����ͭ��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijѧУ����С����ʵ�������Ʊ�Fe(OH)3���壬�����ǣ��ٽ�����ˮ������У������ˮ�еμ� �����������Һ�ʺ��ɫ��ֹͣ���ȡ��ش��������⣺
��1��������еμӵ��Լ���_____________________________��
��2���÷�Ӧ�Ļ�ѧ����ʽΪ_______________________________________________��
��3�����Ƶõ�Fe(OH)3�����в����������Ե缫��ֱͨ����һ��ʱ���������������ɫ____�����������Ϊ______________��
��4�������м������ϡ���ᣬ������������___________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ֶ�����Ԫ�������ڱ��е����λ������ͼ��ʾ������ZԪ��ԭ�Ӻ��������������������������3����
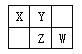
��ش��������⣺
��1��Ԫ��Zλ�����ڱ��е�λ��_____________________��
��2��X��Y��Z����Ԫ�ص����������Ӧˮ������������ǿ��������Ϊ________________��д��ѧʽ����
��3��X��Y�γ�һ���ж��Ķ�Ԫ���������Է���������50~60֮�䣬�������ʽ��X����������ԼΪ46�����û�����ķ���ʽΪ__________���÷����и�ԭ������������8�����ȶ��ṹ����ṹʽΪ ___________.
��4��д��W������������Ӧˮ�����Ũ��Һ��X�ڼ��������·�Ӧ�Ļ�ѧ����ʽ_____________��
��Ӧ����ת��12mol���ӣ����ĵĻ�ԭ������Ϊ______g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Na2S2O3����Ҫ�Ĺ�ҵԭ�ϣ�����С����SO2Ϊԭ����ȡNa2S2O3��ʵ�鲽�����£�
I. ���ƻ����Һ
��Na2S��Na2CO3��2:1�����ʵ���֮�Ȼ�ϣ�����һ������ˮ���γ���Һ��
II.Ԥ�ȡ���ַ�Ӧ
������Һע�볨�ڷ�Ӧ���У����ȷ�Ӧ��һ��ʱ�䣬��Ӧ���л���ͨ��SO2���Թ�����
III.����Ũ������ȴ�ᾧ
��Ӧ���������Ũ����Һ����ȴ��30�����£���������Na2S2O3 5H2O��
IV.���ˡ�ѭ����Ӧ
�˳�Na2S2O3 5H2O�������ȥ�ᾧˮ��ĸҺѭ�����á�
�ش��������⣺
(1)����IIԤ��ʱ�¶Ȳ��˹��ߣ�Ӧ���¶ȿ�����50�����ң�����ҪĿ����____________��
(2)����II��ַ�Ӧʱ�Ļ�ѧ����ʽΪ_______________________��
(3)����IV�е�ĸҺѭ�����ã���Ŀ�����ڽ�Լ�ɱ���������Ⱦ��ͬʱ�����________��
(4)Ϊ�ⶨ����Na2S2O3��Ʒ�Ĵ��ȣ���ȡw g��ɺ��Na2S2O3��Ʒ��������������ˮ�ܽ⣬����a mol L-1��ı���Һ�ζ�(2S2O32-+I2=2I-+S4O62-���Ҳ�����������ⷴӦ)���Ե�����Һ��ָʾ�����ζ����յ�ʱ������Ϊ_________________��
�����ĵ�ı���Һ�����Ϊb mL�������ò�Ʒ�Ĵ���Ϊ_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ��ȥ�����е�Ca2����Mg2����SO42- �Լ���ɳ�����ʣ�ijͬѧ�����һ���Ʊ����ε�ʵ�鷽������������(���ڳ������Լ��Թ���)��
![]()
(1)�ж�BaCl2�ѹ����ķ�����_______________________________________________��
(2)�ڢܲ��У���ص����ӷ���ʽ��___________________________________________��
(3)�����������ٹ��ˣ�����ʵ��������Ӱ�죬��ԭ����______________________________��
(4)Ϊ���龫�δ��ȣ�������150 mL 0.2 mol/L NaCl(����)��Һ����ͼ�Ǹ�ͬѧת����Һ��ʾ��ͼ�����еĴ�����____________________________________________��

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com