
Ϊ�ⶨijFe2O3��FeO�������Fe2O3�ĺ�������һ������Ʒ�м���200 mL��5.0 mol��L��1���ᣬ��ַ�Ӧ�����Һ��c(H+)��1.0 mol��L��1(����Һ�����Ϊ200 mL)���ټ�������2.0 mol��L��1��NaOH��Һ��ʹFe2+��Fe3+��ȫ������������ȫ����Ϊ���ɫ���ˣ�ϴ�ӡ����ճ�����Ƶù���������ԭ��Ʒ����2.4 g��
(1)����Ʒ������Ӧ����������ʵ���Ϊ���٣�
(2)�������ٺ���NaOH��Һ������ʹFe3+��Fe2+ǡ�ó�����ȫ��
(3)�û������Fe2O3�����������Ƕ��٣�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �о�������ұ������������Ҫ���壮
�о�������ұ������������Ҫ���壮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���Ͻ�����Ԫ�ص����ʵ�����0.01 mol | B���Ͻ��������ʵ�������1.68 g | C����ҺA�д��ڵ�������ֻ��Fe2+���� | D��������BΪͭ������������1.6 g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��������Ͽ��������ڶ���ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
I����ͼ��ʾ�ӹ��������з���Q��2�ַ�������ش��й����⡣

��1��ѡ�÷���(i)ʱ��QӦ�þ��е�������_____________��������Ӧ�þ�
�������__________________________________��
��2��ѡ�÷���(ii)��ij������ĩ������Au��Ag��Cu���з���Au��������Լ�Ϊ____________��
��3��Ϊ�ᴿijFe2O3��Ʒ����Ҫ������SiO2.Al2O3�������շ���(i)��(ii)�������һ���Կ�ͼ��ʽ��ʾ��ʵ�鷽����ע�����ʺͲ�������
______________________________________________________________________________��
��ij�ֺ������������ƵĹ���������Ʒ����֪��Ʒ����Ϊ1.560g����ƿ��ˮ������Ϊ
190.720g)����������ͼ��ʾװ�òⶨ�������Na2O2������������ÿ����ͬʱ����õ�
����ƽ���������±���
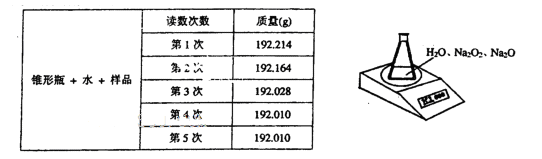
��4��д��Na2O2��H2O��Ӧ�Ļ�ѧ����ʽ��________________________________________��
��5������Na2O2��������ʱ�������������_________________________________________.
��������6�ζ�����ԭ����_____________________________________________________��
��6���ⶨ������Ʒ(1.560g)��Na2O2������������һ�ַ�����������������£�

�����ڵ�������____________���÷�����ֱ�Ӳⶨ����������_____________ ���ⶨ������
��Ҫ�������е�����ƽ�������ƾ��ƣ�����Ҫ___________��__________���̶�����
���������⣩,��ת����Һʱ������Һת�Ʋ���ȫ����Na2O2���������IJⶨ���_______����
��ƫ����ƫС�����䡱����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ�ⶨijFe2O3��FeO�������Fe2O3�ĺ�������һ������Ʒ�м���200 mL 5.0 mol?L��1���ᣬ��ַ�Ӧ�����Һ�����Ϊ200 mL����Һ��c(H+)= 1.0 mol?L��1���ټ�������2.0 mol?L��1NaOH��Һ��ʹFe2+��Fe3+ǡ����ȫ��������ֽ��貢�ȣ�������ȫ����Ϊ���ɫ���ˡ�ϴ�ӡ����ճ�����Ƶù���������ԭ��Ʒ����2.4 g��
��1������Ʒ������Ӧ����������ʵ���Ϊ���٣�
��2���������ٺ���NaOH��Һ������ʹFe2+��Fe3+ǡ�ó�����ȫ��
��3���û������Fe2O3�����������Ƕ��٣�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com