
����Ŀ��ijУ�о���ѧϰС���ͬѧ���������»�ѧʵ�飺�����������ڿ�����ȼ�գ�Ȼ�������ù�������м���һ��������ˮ���˹����з�Ӧ�ų��������ȣ����ҷų��г�ζ�����塣
��1����ͬѧ������������ѧϰ��˼�룬Ca��Mgλ��ͬһ���壬��ѧ���ʾ���һ���������ԡ���д��Ca�ڿ�����ȼ�շ�����Ӧ�Ļ�ѧ����ʽ_______________________________��
��2����ͬѧ�����Ca�����ʱ�Na���ã��ڿ�����ȼ�ջ�Ӧ��CaO2���ɣ���д��ȼ�պ���������ˮ��Ӧ�ų�����Ļ�ѧ����ʽ______________________________����ͬѧ�����ʵ��ķ���̽���ų���ζ����ijɷ֣�
���������ϣ�
1��CaO2��ˮ��Ӧ����H2O2��H2O2���ܻ�ֽ����һ������O3��
2������������õij����ⶨ��������ԭ��Ϊǿ����������(O3)��⻯��(KI)ˮ��Һ��Ӧ���������(I2)������ת��Ϊ��������ӦʽΪO3��2KI��H2O===O2��I2��2KOH��
��������裩
����1���ó�ζ����ֻ��NH3��
����2���ó�ζ����ֻ��________��
����3���ó�ζ���庬��________��
����Ʒ���������ʵ��̽����
��3����С��ͬѧ�������ʵ�鷽����������ʵ�飬��֤�������衣�������ص�ʵ��������衢Ԥ��������(������ѡ)��
��ѡʵ���Լ�����ɫʯ����ֽ����ɫʯ����ֽ��pH��ֽ�����ۣ�KI��Һ������ˮ��
ʵ����� | Ԥ����������� |
ȡ������Ӧ��������Թ��У�_____________ | ______________________________________ |
���𰸡�2Ca��O2![]() 2CaO��3Ca��N2
2CaO��3Ca��N2![]() Ca3N2��2Ca��CO2
Ca3N2��2Ca��CO2![]() 2CaO��C2CaO2��2H2O=2Ca(OH)2��O2����Ca3N2��6H2O=3Ca(OH)2��2NH3��O3O3��NH3���Թ��м�������ˮ����ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ���ȡ������Ӧ��������Թ��У����Թ��м�������ˮ��������������ͨ����ۣ�KI��Һ����ʪ��ĺ�ɫʯ����ֽ����ɫ���ҵ��ۣ�KI��Һ����ɫ�������1��������ʪ��ĺ�ɫʯ����ֽ������ɫ���ҵ��ۣ�KI��Һ����ɫ�������2��������ʪ��ĺ�ɫʯ����ֽ����ɫ���ҵ��ۣ�KI��Һ����ɫ�������3����
2CaO��C2CaO2��2H2O=2Ca(OH)2��O2����Ca3N2��6H2O=3Ca(OH)2��2NH3��O3O3��NH3���Թ��м�������ˮ����ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ���ȡ������Ӧ��������Թ��У����Թ��м�������ˮ��������������ͨ����ۣ�KI��Һ����ʪ��ĺ�ɫʯ����ֽ����ɫ���ҵ��ۣ�KI��Һ����ɫ�������1��������ʪ��ĺ�ɫʯ����ֽ������ɫ���ҵ��ۣ�KI��Һ����ɫ�������2��������ʪ��ĺ�ɫʯ����ֽ����ɫ���ҵ��ۣ�KI��Һ����ɫ�������3����
��������
��1������þ���ԺͿ����е�������������̼��������Ӧ������þ�Ļ�ѧ���ʾ���һ���������ԣ����Ը�Ҳ���Ժ����������巢����Ӧ����Ӧ�Ļ�ѧ����ʽΪ2Ca+O2![]() 2CaO��3Ca+N2
2CaO��3Ca+N2![]() Ca3N2��2Ca+CO2
Ca3N2��2Ca+CO2![]() 2CaO+C����2������ˮ��Ӧ�ų����������ΪCaO2��Ca3N2����ˮ������Ӧ�Ļ�ѧ����ʽ�ֱ�Ϊ2CaO2+2H2O
2CaO+C����2������ˮ��Ӧ�ų����������ΪCaO2��Ca3N2����ˮ������Ӧ�Ļ�ѧ����ʽ�ֱ�Ϊ2CaO2+2H2O![]() 2Ca(OH)2+ O2����Ca3N2+6H2O
2Ca(OH)2+ O2����Ca3N2+6H2O![]() 3Ca(OH)2+2NH3������3����������������Ϣ֪������ζ�����������NH3��Ҳ������O3���������Ƕ��ߵĻ�����֤�Ƿ���NH3������ʪ��ĺ�ɫʯ����ֽ������ֽ��������֤����NH3����֤�Ƿ��г��������õ���KI��Һ���������Ϣ֪������ʹ����KI��Һ���������Թ��м�������ˮ����ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ���ȡ������Ӧ��������Թ��У����Թ��м�������ˮ��������������ͨ�����KI��Һ�� ����ʪ��ĺ�ɫʯ����ֽ����ɫ���ҵ���KI��Һ����ɫ�������1��������ʪ��ĺ�ɫʯ����ֽ������ɫ���ҵ���KI��Һ����ɫ�������2��������ʪ��ĺ�ɫʯ����ֽ����ɫ���ҵ���KI��Һ����ɫ�������3����
3Ca(OH)2+2NH3������3����������������Ϣ֪������ζ�����������NH3��Ҳ������O3���������Ƕ��ߵĻ�����֤�Ƿ���NH3������ʪ��ĺ�ɫʯ����ֽ������ֽ��������֤����NH3����֤�Ƿ��г��������õ���KI��Һ���������Ϣ֪������ʹ����KI��Һ���������Թ��м�������ˮ����ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ���ȡ������Ӧ��������Թ��У����Թ��м�������ˮ��������������ͨ�����KI��Һ�� ����ʪ��ĺ�ɫʯ����ֽ����ɫ���ҵ���KI��Һ����ɫ�������1��������ʪ��ĺ�ɫʯ����ֽ������ɫ���ҵ���KI��Һ����ɫ�������2��������ʪ��ĺ�ɫʯ����ֽ����ɫ���ҵ���KI��Һ����ɫ�������3����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������춡������![]() )��һ�ֺϳ����ϣ�����һ�ֺϳ�·����ͼ:
)��һ�ֺϳ����ϣ�����һ�ֺϳ�·����ͼ:

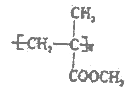
��֪��R-CH=CH2![]() R-CH2CH2OH����ش��������⣺
R-CH2CH2OH����ش��������⣺
��1��A�Ļ�ѧ������______________��B�к��еĹ�����������________________��
��2����һ�������£�A��ˮ��ӦҲ��������B������һ��������÷�Ӧ�ķ�Ӧ����Ϊ___________��������һ��������Ľṹ��ʽΪ_____________________________��
��3��C�ɱ����Ƶ�Cu(OH)2����Һ������Ҳ���Ա�������������������д��C��������Һ��Ӧ�Ļ�ѧ����ʽ��_____________________________��
��4��D�ĺ˴Ź���������ʾΪ6��壬�ҷ����֮��Ϊ1��1��1��1��2��2����D�Ľṹ��ʽΪ_______________��D��ͬ���칹���ж��֣����б�����������������״��ȡ������������Na��Ӧ��ͬ���칹����_______�֣����������칹����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵������ȷ����
A. ��״���£�22.4 Lˮ�������ķ�����ԼΪ6.02��1023��
B. 1 mol Cl2�к��е�ԭ����ΪNA
C. ��״���£�a L�����͵����Ļ���ﺬ�еķ�����ԼΪ![]() ��6.02��1023��
��6.02��1023��
D. ��1 L0.5 mol��L��1NaCl��Һ��ȡ��100 mL��ʣ����Һ��NaCl���ʵ���Ũ��Ϊ0.45 mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��
(1)Ӫ��ƽ�⡢������ҩ�DZ�֤���彡����������������Ҫ;����
�����������У�����Ϊ�����ṩ��������_______(����ĸ)��
a.������ b.��ά�� c.��֬
�����������˸�Ⱦʱ��ҽ���Ὠ��ʹ�����³���ҩ���е�______(����ĸ)��
a.������������Ƭ b.��˾ƥ�� c.��������
��ʳƷ���Ӽ��������Ƶ������ʳ�β�����ζ���������Ƿ������������п��������á�������������____(����ĸ)�����������ƻ�������ĵ����ʷ�Ӧ������һ���°����������������������Բ��ɳ��ڻ������ʳ���������ࡣ
a.��ζ�� b.��ɫ�� c.��ɫ��
(2)����������̬������ʵ��������Ȼ�ĺ�г������
����ú�м���ʯ��ʯ������Ч����_______���ŷš�
������Hg2+�ķ�ˮ�м���____������Ч��ȥ���ؽ������ӡ�
�����������ܸ�ˮ��ɱ������������ʹˮ�徻������______(����ĸ)��
A.Na2FeO4(aq) B.KAl(SO4)2��12H2O C.Ca(ClO)2(aq) D.NaClO(aq)
���ö�����̼����������Ʒ�������ڶ�����̼�Ĵ������ա�ij��ҵ����������CO2��C2H4��ˮ�����ڴ��������ºϳ�����(ԭ��������100%)���÷�Ӧ�Ļ�ѧ����ʽΪ____________��
(3)��������������ͷ�չ�����ʻ�����
��������ʻ�ڡ��������ٹ�·������ʻ���Զ��շ�ϵͳ��ͨ������оƬ�Գ��������Զ��շѡ���ȡоƬ����Ҫԭ����_____ (����ĸ)��
a.�� b.ʯī c.��������
�������в����У����ڸ��ϲ��ϵ���____ (����ĸ)��
a.�ֻ����� b.������ c.�������մ� d.�ֽ������
������������Ҫ������ˮ�࣬ˮ������______(����ĸ)��ˮ��ı�����ͨ��ֻ�������£����ܳ��ڱ����ԭ����_______________��
a.�������� b.���ǽ������� c.�л��߷��Ӳ���
���л����������л���X�Ӿ��Ƶõ����������ϣ�Ϊ���粣��״����ɫ���壬���������캽�մ��������DZ��̡���������ơ�װ������������Ʒ�ȣ���ṹ��ʽ��ͼ��ʾ����д��X �Ľṹ��ʽ__________��
��̫���ܵ����Ҫ�õ��ߴ���Ϊԭ�ϡ������½�̿��ʯӢ��Ӧ�����Ƶôֹ裬�÷�Ӧ�ķ���ʽΪ__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ij��ѧ��ȤС����Ƶ����õ�������(��70% Cu��25% Al��4% Fe������Au��Pt)�Ʊ�����ͭ�������������·�ߣ�

��֪������Ϣ��Cu����ϡ�����H2O2�Ļ��Һ��Ӧ��������ͭ����������ͭ������������������ʽ����ʱ��Һ��pH���±���
������ | Fe(OH)3 | Al(OH)3 | Cu(OH)2 |
��ʼ���� | 1.1 | 4.0 | 5.4 |
��ȫ���� | 3.2 | 5.2 | 6.7 |
��1��д��Cu��ϡ�����H2O2�Ļ��Һ��Ӧ�Ļ�ѧ����ʽ��_________________________________��
��2���ڲ������У�x��ȡֵ��Χ��____________��
��3���ڲ������У�����Ũ����Ҫ�IJ���������________________��
��4��������a��ȡAl2(SO4)3��18H2O��̽��С����������ַ�����
�ף�����a�D��H2SO4�D��������D��Al2(SO4)3��18H2O
�ң�����a�D��H2SO4�D������Al�ۣ����˨D��������D��Al2(SO4)3��18H2O
��������a�D��NaOH��Һ�����˨D��H2SO4�D��������D��Al2(SO4)3��18H2O
�ۺϿ����������ַ�������߿����Ե���______(�����)��
��5��Ϊ�ⶨCuSO4��5H2O����Ĵ��ȣ���������ʵ�飺ȡa g �������100 mL��Һ��ÿ��ȡ20.00 mL�������������Ӻ���b mol��L��1 EDTA(Na2H2Y)����Һ�ζ����е�Cu2��(���ӷ���ʽΪCu2����H2Y2��===CuY2����2H��)���ζ����յ㣬ƽ������EDTA��Һ12.00 mL(����ʱ��5%��Na2H2Yˮ��Һ����pHΪ4��6)����CuSO4��5H2O����Ĵ�����________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ᴿ������ͼװ�ý��У���Ҫ��ش��������⣺
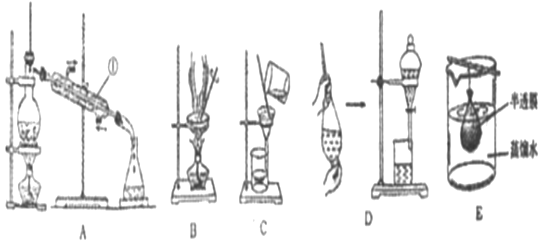
��1�������ٵ�����________________________��
��2��������ˮ���Ե����ʻ�������ˮ�γɽ��塣����������ˮ��Һ��Na2CO3��Na2SO4�Ļ����ҺӦѡ��װ�õ�Ϊ������ͼ��ĸ��д��____________________�����֤��SO42-�ѷ������______________________��
��3����װ��D�м���10 mL��ˮ��Ȼ����ע��4 mL�����Ǻò��������������������ù۲쵽�������ǣ�________________________��������Ϻ�Ϊ�õ��Ⲣ���ձ�����______________����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijǿ������ҺX�п��ܺ���Fe2+��A13+��NH4+��CO32-��SO32-��SO42-��Cl-�е������֣���ȡX��Һ��������ʵ�飬ʵ����̼��������£�����˵������ȷ����

A. X �п϶����� Fe2+��A13+��NH4+��SO42-
B. X�в���ȷ���Ƿ���ڵ�������Al3+��Cl-
C. ��ҺE������F������Ӧ��������Ϊ����
D. ����A��NO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ȤС���ͬѧΪ���Ʊ�������̽�������ʣ��ش��������⡣
��д��ʵ������ȡ���������ӷ���ʽ��_______________________________
��ͬѧ�����ͼ��ʾװ���о������ܷ���ˮ������Ӧ������a�Ǻ�������������ˮ��������������ش��������⣺

��1��Ũ�����������___________________��
��2��֤��������ˮ��Ӧ��ʵ������Ϊ__________��
��3��ICl��������Cl2���ƣ�д��ICl��ˮ��Ӧ�Ļ�ѧ����ʽ��______________________��
��4����������ͨ��ʯ������ȡƯ�ۣ���Ӧ�Ļ�ѧ����ʽ��______________________��Ư������ˮ�����������е�CO2��������Ư�ס�ɱ�����ã���Ӧ�Ļ�ѧ����ʽ��_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ʹ�����CO2�ĺ�������Ч�ؿ�������CO2����������ȫ������ձ�����
��1��CO2����ϳ�DME�������ѣ��ǽ����ԴΣ�����о�����֮һ��
��2CO2(g) + 6H2(g)![]() CH3OCH3(g) + 3H2O(g) ��H= -122.4kJ��mol-1
CH3OCH3(g) + 3H2O(g) ��H= -122.4kJ��mol-1
ij�¶��£���2.0 mol CO2(g) ��6.0 mol H2(g)�����ݻ�Ϊ2 L���ܱ������У���Ӧ����ƽ��ʱ���ı�ѹǿ���¶ȣ�ƽ����ϵ�� CH3OCH3(g) �����ʷ����仯�����ͼ��ʾ����P1_______P2���>����<����=������ͬ������T1��P1��T3��P3ʱƽ�ⳣ���ֱ�ΪK1��K3����K1________K3��T1��P1ʱH2��ƽ��ת����Ϊ______________��

���ں����ܱ������ﰴ�����Ϊ1��3���������̼��������һ�������·�Ӧ�ﵽƽ��״̬�����ı䷴Ӧ��ijһ��������������˵��ƽ��һ�����淴Ӧ�����ƶ�����______������ţ���
A����Ӧ���Ũ������ B�����������ܶȼ�С
C������Ӧ����С���淴Ӧ���� D��������ת���ʼ�С
��2����һ������CO2����ͨ���������Ƶ���Һ�У���������Һ�бߵμ�ϡ�������������������������������������ʵ����Ĺ�ϵ��ͼ������������ܽ��HCl�Ļӷ�������ش𣺵�����HCl �����ʵ���Ϊ1 molʱ����Һ���������ʵĻ�ѧʽ__________��a����Һ�и�����Ũ���ɴ�С�Ĺ�ϵʽΪ____________________________________��

��3��CO2����Ȼ��ѭ��ʱ����CaCO3��Ӧ��CaCO3��һ���������ʣ���Ksp = 2.8��10��9��CaCl2��Һ��Na2CO3��Һ��Ͽ��γ�CaCO3�������ֽ��������CaCl2��Һ��Na2CO3��Һ��ϣ������ǰNa2CO3��Һ��Ũ��Ϊ2��10��4 mol��L��1�������ɳ�������CaCl2��Һ����СŨ��Ϊ___________ mol��L��1��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com