
| c(CH3OH)?c(H2O) |
| c(CO2)?c3(H2) |
| c(CH3OH)?c(H2O) |
| c(CO2)?c3(H2) |
| 3 |
| 2 |
| 3 |
| 2 |
| ��c |
| ��t |
| 1mol��32g/mol |
| 16g |
| 0.75mol/L |
| 10min |
| 2.25mol |
| 3mol |
| c(CH3OH)?c(H2O) |
| c(CO2)?c3(H2) |
| c(CH3OH)?c(H2O) |
| c(CO2)?c3(H2) |
| 1mol��32g/mol |
| 16g |
| 3 |
| 2 |
| 3 |
| 2 |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
| ���� |
| ���� |
| ���� |
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| C(CH3OH)?C(H2O) |
| C(CO2)?C3(H2) |
| C(CH3OH)?C(H2O) |
| C(CO2)?C3(H2) |
| 3 |
| 2 |
| 3 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014������ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
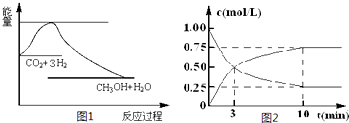
ʵ�� �����ܼ��š� �͡���̼���á���һ����Ҫ���������ν�CO2ת��Ϊ�����õ���Դ��Ŀǰ��ҵ����һ�ַ�������CO2������ȼ�ϼ״���һ�������·�����Ӧ��
 CO2(g)+3H2(g)
CO2(g)+3H2(g) CH3OH(g)+H2O(g)����ͼ1��ʾ�÷�Ӧ�����������仯��
CH3OH(g)+H2O(g)����ͼ1��ʾ�÷�Ӧ�����������仯��
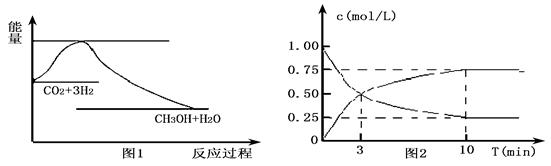
��1�����ڸ÷�Ӧ������˵���У���ȷ����____________(����ĸ)��
A����H��0����S��0 B����H��0����S��0
C����H��0����S��0 D����H��0����S��0
��2��Ϊ̽����Ӧԭ�����ֽ�������ʵ�飬�����Ϊl L���ܱ������У�����l mol
CO2��4mol H2��һ�������·�����Ӧ��CO2(g)+3H2(g) CH3OH(g)+H2O(g)�����CO2��CH3OH(g)��Ũ����ʱ��仯����ͼ2��ʾ��
CH3OH(g)+H2O(g)�����CO2��CH3OH(g)��Ũ����ʱ��仯����ͼ2��ʾ��
�ٴӷ�Ӧ��ʼ��ƽ�⣬CO2��ƽ����Ӧ����v(CO2)�� ��
H2��ת����w(H2) = ��
�ڸ÷�Ӧ��ƽ�ⳣ������ʽK�� ��
�����д�ʩ����ʹ��ѧƽ��������Ӧ�����ƶ����� (����ĸ)��
A�������¶�
B����CH3OH(g)��ʱҺ�����
C��ѡ���Ч����
��3��25�棬1.01��105Paʱ��16gҺ̬�״���ȫȼ�գ����ָ���ԭ״̬ʱ���ų�363.3kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ��_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ��ɽ����ѧ���и���2���¿������ۣ���ѧ���� ���ͣ�ѡ����
��ҵ���Ի�����Ϊԭ���������ᡣ
��1��д��ȼ�ջ�����Ļ�ѧ����ʽ ��
��2���Ӵ����з�����Ӧ�Ļ�ѧ����ʽ�� ��
��3���ж�����˵����ȷ����(ѡ�������ĸ) ��
a��Ϊʹ��������ȼ�գ��轫�����
b���������������SO2��ת����
c��ʹ�ô��������SO2�ķ�Ӧ���ʺ�ת����
d������¯�ų��Ŀ����ɹ�����
��4������������Ũ���������������������ˮ��ԭ������ ��
��5���������ų���β�����������ַ���������
�����٣����ð�ˮ���գ�����Ũ���ᴦ������Ũ���ᴦ������ҪĿ���� ��
�����ڣ����ú�һ��ˮ�������������պ��ټ��ȴ�����Ҳ�ɴﵽ�뷽������ͬ��Ŀ�ġ�Ϊ��ʵ�ֽ��ܼ��ţ����������������� �ṩ���뷽������ȣ������ڵ��ŵ��� ��
��6��SO2 �ȿ���Ϊ���������ԭ��ѭ�������ã�Ҳ�����ڹ�ҵ������������ճ�ʪ�����е�Br2��SO2 ����Br2�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com