
Ԫ�����ڱ��еڢ�A��Ԫ�صĵ��ʼ��仯�������;�㷺��
��1������Ϊ�ȡ��塢��Ԫ�طǽ�����(ԭ�ӵõ�������)�ݱ���ɵ��ж������� (�����)��
a��Cl2��Br2��I2���۵� b�� Cl2��Br2��I2��������
c��HCl��HBr��HI�����ȶ��� d�� HCl��HBr��HI������
��2����ҵ�ϣ�ͨ������ת�����Ƶ�KClO3���壺
NaCl��Һ NaClO3��Һ
NaClO3��Һ KClO3����
KClO3����
�����I�з�Ӧ���ܻ�ѧ����ʽ����NaCl����H2O����NaClO3���� ��
�ڢ���ת���Ļ�����Ӧ������ ���÷�Ӧ����������KClO3���������������������ԭ���� ��
��3��һ����������ˮ��Һ��1 mol Cl����ClOX��(x��1��2��3��4)������(KJ)��Դ�С����ͼ��ʾ��
��D�� (�����ӷ���)��
��B��A��C��Ӧ�����ӷ���ʽΪ ������1molCʱ�� KJ���ȣ������ջ�ų��Լ�������ֵ��
��1��b��c ��2�֣�
��2����1NaCl��3H2O��1NaClO3��3H2�� ��2�֣�
�ڸ��ֽⷴӦ �����£��������ˮ�е��ܽ������С���������壨2�֣�
��3����ClO4�� ��1�֣� ��3ClO����ClO3�� ��2Cl������2�֣� �ų�117��1�֣�
�������������ͬһ����Ԫ�أ�Ԫ�صķǽ�����Խǿ�����⻯����ȶ���Խǿ��������������ˮ��������Խǿ���䵥�ʵ�������Խǿ�������ʵķе㡢�⻯��ˮ��Һ�������أ���ѡbc�����ʱ�������������ӷŵ�������������ӡ������������ӷŵ��������������Է�Ӧ����ʽΪ1NaCl+3H2O�T1NaClO3+3H2�����ʴ�Ϊ��1��3��1��3
NaClO3ת��ΪKClO3��˵���÷�Ӧ������������������������Σ�Ϊ���ֽⷴӦ����ͬ�¶��£��ܽ��С��������������������KClO3��ˮ�е��ܽ������С���������壬����������KClO3���ʴ�Ϊ�����ֽⷴӦ��������KClO3��ˮ�е��ܽ������С���������壻
����ͼ��֪��D��ClԪ�ػ��ϼ�Ϊ+7�ۣ�����ClOx-��xΪ4����DΪClO4-���ʴ�Ϊ��ClO4-��
��B��A+C������ת�Ƶ����غ�ø÷�Ӧ����ʽΪ3ClO-=ClO3-+2Cl-����Ӧ��=��63kJ/mol+2��0kJ/mol��-3��60kJ/mol=-117kJ/mol�����Ը��Ȼ�ѧ��Ӧ����ʽΪ3ClO-��aq��=ClO3-��aq��+2Cl-��aq����H=-117kJ/mol���ʴ�Ϊ��3ClO-��aq��=ClO3-��aq��+2Cl-��aq����H=-117kJ/mol��
���㣺������±��Ԫ��Ϊ���忼����������ԭ��Ӧ���Ȼ�ѧ��Ӧ���ǽ���ǿ�����жϷ�����֪ʶ��


 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
14gCu��Ag�Ͻ�������ijŨ�ȵ����ᷴӦ�����ų���������1.12L O2(���)��ϣ�ǡ���ܱ�ˮȫ�������������ᣬ��Ͻ���Cu������Ϊ
| A��1.6g | B��3.2g | C��6.4g | D��9.6g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�������й����ʵ����ʻ���;��˵������ȷ����
�� ��������Ư���ԣ�����ֱ��ʹ��ɫ������ɫ���� ����������н�ǿ�Ļ�ԭ�ԣ�������Ũ�������� SiO2�������������������ᷴӦ���� Al(OH)3�������������������ǿ���ǿ�
| A���٢ڢ� | B���ڢۢ� | C���ۢ� | D���٢ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
������һ����Ҫ�Ĺ�ҵԭ�ϡ���ҵ�����÷�Ӧ��3Cl2+8NH3=N2+6NH4Cl��������ܵ��Ƿ�©��������˵����ȷ����
| A�����ܵ�©�������ͻ�������� | B���÷�Ӧ�����˰����Ļ�ԭ�� |
| C���÷�Ӧ���ڸ��ֽⷴӦ | D������6molNH4Cl��18mol����ת�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ����Ư�۵Ļ�ѧ����ʽΪ��______________________________________;Ư����ˮ�������տ����е�CO2��ʵ��Ư��ԭ��:____________________________(�û�ѧ����ʽ��ʾ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��10�֣�A��H��8�����ʴ�������ת����ϵ(��Ӧ���������ֲ���δ���)����֪A�����Σ�B����ʹƷ����Һ��ɫ�����壬G�Ǻ���ɫ���塣��Ҫ��ش����⣺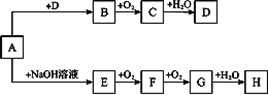
��1��д���������ʵĻ�ѧʽ��A ��B ��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ��E��F ����
д�����з�Ӧ�����ӷ���ʽ��A��E ��
H��Ũ��Һ��ľ̿��Ӧ�Ļ�ѧ����ʽ�� ��
��3������ij��Һ���Ƿ�D�������ӵķ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(10��)(I) �����к��е�Ԫ�أ�ijУ�о���ѧϰС�����������ʵ�鲽������ȡ�⣺
������Һ�У��μӼ��������������˫��ˮ �ڽ������ճɻң�����м�ˮ�����Ƚ���
�ۼ�CC14������ �ܹ��� �ݷ�Һ��
��1�������IJ���˳��Ϊ ��
��2���������Ҫ�õ��IJ�������Ϊ ���ò�����I2�IJ����� ��
(II)��ij����Fe2+��I����Br������Һ�л���ͨ��������������Һ�и������ӵ����ʵ����仯��ͼ��ʾ��
��3��AB�α�ʾ ���ӵļ��١�
��4��n(Cl2)=2molʱ����Һ�з�Ӧ�����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����仯ѧ�����ս����̿�(��Ҫ�ɷ�MnO2)��Ũ�����ϼ��ȣ��������������Ƶ���������д���÷�Ӧ�����ӷ���ʽ ��
��2�����ֹ�������ˮ������ɱ����Ϊ���ƴ��ģ��Ⱦ�Լ�����������Ч����֮һ�� Ư���dz��õ�����������ҵ�Ͻ�����ͨ��ʯ����[Ca(OH)2]��ȡƯ�ۣ���ѧ��Ӧ����ʽΪ ��
��3������ͼ������ֱ�߷ֱ��ʾ�ơ�ͭ��������������Cl2��Ӧʱ�����Ľ���������(����)�뷴Ӧ������������(����)�Ĺ�ϵ�����д�������Cl2��Ӧ��ֱ���� ����������ʾ���ĵ��������������b��ʾ�������ֽ����е� ��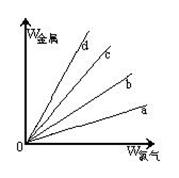
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com