
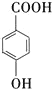
����Ŀ�����������������մɵ������Ļ��塢���Ϻʹ�������ҵ����������(��Ҫ��Na2SnO3��Na2TeO3)Ϊԭ�ϣ��Ʊ������ƵĹ�������ͼ���£���ش��������⣺
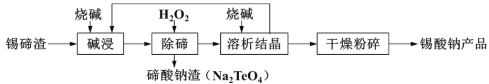
��֪��������(Na2SnO3)����������(Na2TeO3)�������ڼ
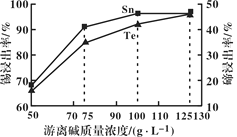
(1)������������У����ڽ���������Һ�����������Ũ�ȹ�ϵ��ͼ��ʾ���������������Ũ��Ϊ__________��������______________��
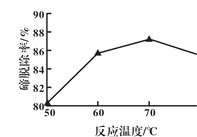
(2)��ͼ��ӳ�����������������з�Ӧ�¶ȶ����ѳ��ʵ�Ӱ���ϵ��70������¶��������ѳ����½���ԭ�������______________��
(3)����������Ӧ�����ӷ���ʽΪ_________________��
(4)���������ᾧ���ص�������������ʳ��ռ��⣬��Ҫ����_____________(д��ѧʽ)��
(5)�������ᾧ��ĸҺ�л���������SbO43-��������Ƭ��Sb�û���������ת������ۺ������Σ�д����Ӧ�����ӷ���ʽ___________________��
���𰸡�100g/L Ũ�ȳ���100����������������С�����ڽ�����ȴ��߽ϴ����ں������� �¶����ߣ������������ȷֽ� 2Na++TeO32-+H2O2��Na2TeO4��+H2O Na2SnO3��Na2TeO4 5Sn + 4SbO43- + H2O��4Sb + 5SnO32- + 2OH-
��������
(1)������ĵ�Ӧ��������ʸ����������ʣ�
(2)�������������ֽ⣻
(3)�����������ǿ�����ԣ�
(4)�����ᾧ�����Һ��ΪNa2SnO3��Na2TeO4�ı�����Һ��
(5)������ۺ�������ΪH2SnO3����ϵ����غ��Ԫ���غ���ƽ����ʽ��
(1)����ͼ���֪Ũ�ȳ���100����������������С�����ڽ�����ȴ��߽ϴ����ں������룬�ʴ�Ϊ��100g/L��Ũ�ȳ���100����������������С�����ڽ�����ȴ��߽ϴ����ں������룻
(2)�ù��̷�Ӧ�����й������⣬�������������ֽ⣬�ʴ�Ϊ���¶����ߣ������������ȷֽ⣻
(3)����������������ԣ���ԭ����һ��Ϊˮ���ù����й������⽫TeO32-������Na2TeO4�����ݵ����غ��Ԫ���غ��֪����ʽΪ��2Na++TeO32-+H2O2��Na2TeO4��+H2O��
(4)�����ᾧ�����Һ��ΪNa2SnO3��Na2TeO4�ı�����Һ�����Դ��������ᾧ���ص�������������ʳ��ռ��⣬��Ҫ����Na2SnO3��Na2TeO4���ʴ�Ϊ��Na2SnO3��Na2TeO4��
(5)������ۺ�������ΪH2SnO3����Ƭ�ɽ�Sb�û�����������������Sb���ʣ��ٽ�ϵ����غ��Ԫ���غ��֪����ʽΪ��5Sn + 4SbO43- + H2O��4Sb + 5SnO32- + 2OH-��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���״����ӽ���Ĥȼ�ϵ���н��״�����ת��Ϊ���������ַ�Ӧԭ����
��CH3OH��g����H2O��g����CO2��g����3H2��g�� ��H����49.0 kJ/mol
��CH3OH��g����1/2O2��g����CO2��g����2H2��g�� ��H����192.9 kJ/mol
����˵��������ǣ� ��
A.CH3OHת���H2�ķ�Ӧ��һ��Ҫ��������
B.1mol CH3OH��g����ȫȼ�շų�����������192.9 kJ
C.��Ӧ���е������仯��ͼ��ʾ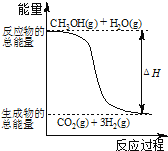
D.���ݷ�Ӧ�ٺ͢���֪��H2��g����1/2O2��g����H2O��g�� ��H=��241.9kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������緶Χ�ڵ���ԴΣ�����״���Ϊһ�ֿ�������Դ���й㷺��Ӧ��ǰ����
(1)��֪�ڳ��³�ѹ�·�Ӧ���Ȼ�ѧ����ʽ��
��CO(g)��2H2(g) CH3OH(g) ��H1����90 kJ��mol��1
��CO(g)��H2O(g) CO2(g)��H2(g) ��H2����41 kJ��mol��1
д���ɶ�����̼�������Ʊ��״����Ȼ�ѧ����ʽ��____
(2)����CO(g)��2H2(g)CH3OH(g) ��H1����90 kJ��mol��1�����ݻ�ΪV L�������г���a mol CO��2a mol H2���ڴ��������·�Ӧ���ɼ״���ƽ��ʱCO��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
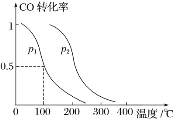
��p1 _______(��������������������������) p2��
���������������������£���С���ʹѹǿ���ﵽ��ƽ��ʱ��CO��ת����________(��������������С������������)��ƽ�ⳣ��________(��������������С������������)��
(3)��֪���¶�Tʱ��CO(g)��H2O(g) CO2(g)��H2(g)��ƽ�ⳣ��K��0.32���ڸ��¶��£���֪cʼ(CO)��1 mol��L��1��cʼ(H2O)��1 mol��L��1��ijʱ�̾��ⶨCO��ת����Ϊ10%���жϴ�ʱ������________(����>�� ����=������<��)������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2015��ŵ��������ѧ��ҽѧ����һ�������ҹ�ҩ�ﻯѧ�������ϣ��Ա�����������ű����ҩ�����غ�˫�������ء���������ɴ�Ϊ��ʼԭ�����˹�ȫ�ϳ������ص�;��֮һ����ͼ��������˵����ȷ����

A.������ɴ��ķ���ʽΪC10H17O
B.������ɴ���NaOH����Һ�пɷ�����ȥ��Ӧ
C.�����ط����к���7������̼ԭ��
D.���������ȵ��ᡢ����Һ�о����ȶ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��[��ѧ�����л���ѧ����]
ij��ˮ�����������ά������Ҫ����ԭ�ϣ����ǵĺϳ�·�����£�
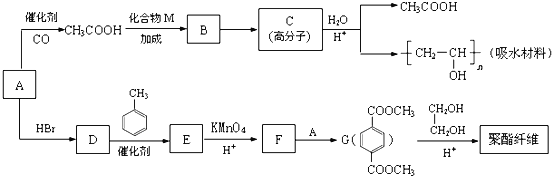
��֪�����л���A����Na��Ӧ����Է�������Ϊ32��
��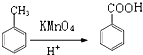
��RCOOR��+R��OH![]() RCOOR��+R��OH��R��R��R������������
RCOOR��+R��OH��R��R��R������������
��1��A�Ľṹ��ʽ��____________��B�к��������ŵ�������_____________��
��2��C�Ľṹʽ��_______________��D��E�ķ�Ӧ������_________________��
��3��F+A��G�Ļ�ѧ����ʽ��_________________________��
��4��CH3COOH+CH��CH��B�Ļ�ѧ����ʽ��______________��
��5��G��ͬ���칹���ж��֣��������������Ĺ���___________�֡�
��������ֻ������ȡ����
��1mol��������NaHCO3��Һ��Ӧ����2mol CO2����
��6��G��������ά�Ļ�ѧ����ʽ��_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���üױ�����һ�ֳ��õĻ�ױƷ��ù����������������ͼ��ʾ![]() ��Ӧ����û��ȫ��ע��
��Ӧ����û��ȫ��ע��![]() ��
��
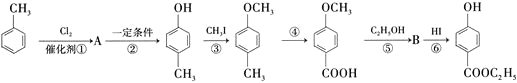
�ش��������⡣
(1)�л���A�Ľṹ��ʽΪ_____________��
(2)�ںϳ�·���У���Ƶڢ۲���Ӧ��Ŀ����_________________________________��
(3)д����Ӧ�ݵĻ�ѧ����ʽ��__________________________________��
(4)����� ����
����![]() �Ļ�ѧ��Ӧ����ʽ��_________________��
�Ļ�ѧ��Ӧ����ʽ��_________________��
(5)�����й�˵����ȷ����_________![]() �����
�����![]() ��
��
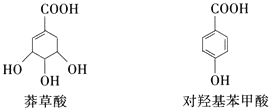
![]() ç���ᡢ���ǻ������ᶼ���ڷ��㻯����
ç���ᡢ���ǻ������ᶼ���ڷ��㻯����
![]() ç������NaOH��Һ��Ӧ���������
ç������NaOH��Һ��Ӧ���������![]()
![]() ���߾����Է����������ӳɵȷ�Ӧ
���߾����Է����������ӳɵȷ�Ӧ
![]() ����
����![]() ��Һ������ç����Ͷ��ǻ�������
��Һ������ç����Ͷ��ǻ�������
(6)д��ͬʱ��������Ҫ��� ������ͬ���칹��Ľṹ��ʽ��_________ ��________��
������ͬ���칹��Ľṹ��ʽ��_________ ��________��
a. ������ ![]() ����
����![]() ��Һ������ɫ��Ӧ c. ������һ�ȴ���������
��Һ������ɫ��Ӧ c. ������һ�ȴ��������� ![]() �ܷ���������Ӧ�����ܷ���ˮ�ⷴӦ
�ܷ���������Ӧ�����ܷ���ˮ�ⷴӦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����б�ʾ���ʵĻ�ѧ������ȷ���ǣ� ��
A.![]() C��ʾ���Ǻ�����13�����ӣ�������8������
C��ʾ���Ǻ�����13�����ӣ�������8������
B.H3O+��OH-������ͬ���������͵�����
C.NaOH�ĵ���ʽ��![]()
D.HClO�Ľṹʽ��H��Cl��O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������л����У�����˳���칹�����( )
A.2-��-1-��ϩB.3-��-1-��ϩ
C.2-��-2-��ϩD.4-��-2-��ϩ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ�����ñ���Ũ���ᡢŨ���ᷢ����Ӧ��ȡ��������װ������ͼ��ʾ����ش��������⣺
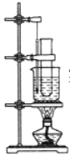
(1)��Ӧ����55��60 ����¶��½��У�ͼ�и���Ӧ����ȵķ�����________��
(2)�����ƻ����ʱ�ȼ�_____________���_____________��
(3)�÷�Ӧ�Ļ�ѧ����ʽ��_____________________�� ��Ӧ����Ϊ��_______��
(4)��Ӧ��Ϻ���ȥ����ᣬ���ôֲ�Ʒ�����²������ƣ�������ˮϴ�����ø�����������10% NaOH��Һϴ����ˮϴ ��ȷ�IJ���˳����________��
A���٢ڢۢܢ� B���ڢܢݢۢ�
C���ܢڢۢ٢� D���ڢܢ٢ݢ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com