
���� ��ɫ��Һ��֪һ������Fe2+��MnO4-���ɵ���غ��֪һ����������ΪNa+��
����һ��ȡ��������Һ����������ϡ���ᣬ�����д̼�����ζ������A����ҺB��������AΪ��������һ����SO32-��
���������B�м�������BaCl2��Һ���ð�ɫ��������ҺC����ɫ����Ϊ���ᱵ����֪һ����SO42-��
����������C��Һ��ͨ������Cl2���ûƺ�ɫ��ҺD��D�к��ⵥ�ʣ���ԭ��Һһ����I-���Դ������
��� �⣺��ɫ��Һ��֪һ������Fe2+��MnO4-���ɵ���غ��֪һ����������ΪNa+��
����һ��ȡ��������Һ����������ϡ���ᣬ�����д̼�����ζ������A����ҺB��������AΪ��������һ����SO32-��
���������B�м�������BaCl2��Һ���ð�ɫ��������ҺC����ɫ����Ϊ���ᱵ����֪һ����SO42-��
����������C��Һ��ͨ������Cl2���ûƺ�ɫ��ҺD��D�к��ⵥ�ʣ���ԭ��Һһ����I-��
��1��������������֪��һ��������ΪSO32-��I-��SO42-��Na+���ʴ�Ϊ��SO32-��I-��SO42-��Na+��
��2������Һ�п��ܴ��ڵ���������CO32-��Cl-���ʴ�Ϊ��CO32-��Cl-��
��3���������ⵥ�ʱ���������������Һ��ƺ�ɫ����Ϊ������ij�����ʣ�ȷ�ϸ����ʵ�ʵ������������ǣ�ȡ����D��Һ���Թ��У����������Һ����Һ������
�ʴ�Ϊ�����������Һ����Һ������
���� ���⿼��������ƶϣ�Ϊ��Ƶ���㣬�������ӵ����ʡ������ķ�ӦΪ���Ĺؼ������ط������ƶ������Ŀ��飬ע��Ԫ�ػ�����֪ʶ��Ӧ�ã���Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 9.2g�����У�������ѧ������ĿΪ0.8 NA | |
| B�� | �����£�28g Fe��������Ũ���ᷴӦ��ת�Ƶĵ�����Ϊ1.5NA | |
| C�� | ��״���£�2.24LNH3��CH4�Ļ�����壬������������ΪNA | |
| D�� | ��״���£�22.4L���������еķ�����ĿΪNA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 10mL pH=13�İ�ˮ��10mL pH=l�������ϣ�c��Cl-����c��NH4+����c��OH-����c��H+�� | |
| B�� | l0 mL0.1 mol/L NH4Cl��Һ��5mL 0.2mol/LNaOH��Һ��ϣ�c��Cl-��=c��Na+����c��OH-������H+�� | |
| C�� | 10 mL 0.1 mol/L CH3COOH��Һ��10 mL pH=13��NaOH��Һ��ϣ�c��CH3COO-��=c��Na+����c��OH-����c��H+�� | |
| D�� | l0 mL 0.5mol/L CH3COONa��Һ��6mL pH=0�������ϣ�c��Cl-����c��Na+����c��OH-����c��H+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��c��CO32-��=0.1 mol•L-1����Һ�У�K+��AlO2-��Cl-��NO3- | |
| B�� | �ڳ�������ˮ�������c��OH-��=1��10-12 mol•L-1����Һ�У�Fe2+��ClO-��Na+��SO42- | |
| C�� | �ڼ��뱽�ӻ�����ɫ����Һ�У�NH4+��Cl-��Na+��SCN- | |
| D�� | ����ʹ��ɫʯ����ֽ������Һ�У�S2O32-��CO32-��Na+��K+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
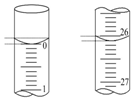 ijѧ����0.100 0mol/L��NaOH����Һ�ζ�δ֪Ũ�ȵ����ᣬ������ɷ�Ϊ���¼�����
ijѧ����0.100 0mol/L��NaOH����Һ�ζ�δ֪Ũ�ȵ����ᣬ������ɷ�Ϊ���¼�����| �ζ� ���� | ������Һ/mL | 0.100 0mol•L-1NaOH�������mL�� | ||
| �ζ�ǰ | �ζ� | ��Һ���/mL | ||
| ��һ�� | 25.00 | 0.00 | 26.11 | 26.11 |
| �ڶ��� | 25.00 | 1.56 | 30.30 | 28.74 |
| ������ | 25.00 | 0.22 | 26.31 | 26.09 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 �����£���100mL 0.01mol•L-1��HA��Һ����μ���0.02mol•L-1��MOH��Һ��ͼ����ʾ���߱�ʾ�����Һ��pH�ı仯���������˵����ȷ���ǣ�������
�����£���100mL 0.01mol•L-1��HA��Һ����μ���0.02mol•L-1��MOH��Һ��ͼ����ʾ���߱�ʾ�����Һ��pH�ı仯���������˵����ȷ���ǣ�������| A�� | HAΪ���� | |
| B�� | �����£�MA��Һ��pH��7 | |
| C�� | K���Ӧ����Һ�У�c��M+��+c��MOH��=c��A-�� | |
| D�� | ��N��K������һ���Ӧ����Һ�У�c��M+��+c��H+��=c��OH-��+c��A-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
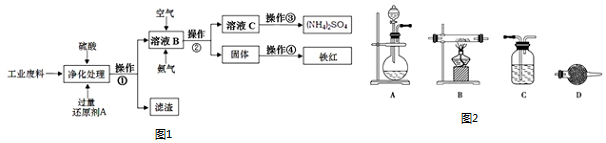
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com