
����Ŀ��(1)�����ֻ��Է�Ӧ�м������ӣ����ǵ������о�����1��̼ԭ�Ӻ�3����ԭ�ӡ�����������������������������ģ�ͣ�д����Ӧ�Ļ�ѧʽ��
 ___________��
___________�� ______________��
______________��
(2)��Ҫ��д���ڶ����ڷǽ���Ԫ�ع��ɵ����Է��ӵĻ�ѧʽ��
ƽ�������η���___________�������η���____________�����������η���_____________��
(3)д��SO3�ij����ĵȵ�����Ļ�ѧʽ��һ��������____________ (д��һ�֣���ͬ)������������____________�����ǵ�����ԭ�Ӳ��õ��ӻ���ʽ����____________��
���𰸡�CH3+ CH3- BF3 NF3 CF4 NO3- CO32- sp2
��������
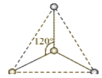
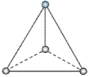
(1)���ݷ��ӵĿռ乹�͡��ӻ������Լ��۲���Ӷ�����������о�����1��̼ԭ�Ӻ�3����ԭ���������жϣ�
(2)�ɵڶ����ڷǽ���Ԫ�ع��ɵ����Է��ӣ��ڶ�����Ԫ��Ϊ����ԭ�ӣ������ƽ���η��ӣ���ͨ��sp2�ӻ��γ����Է��ӣ�����������ͷ��ӣ���ͨ��sp3�ӻ��γ����Է��ӣ��Ҽ۲���ӶԸ�����4������һ���µ��Ӷԣ��������������ṹ����÷��ӵļ۲���ӶԸ�����4�Ҳ����µ��Ӷԣ��ݴ˷������
(3)�ȵ�������ָ������ͬ�۵���������ԭ�������ķ��ӻ����ӣ����ݼ۵��ӶԻ�������ȷ��ԭ�ӵ��ӻ���ʽ���۲���ӶԸ���=��������+�µ��ӶԸ��������жϡ�
(1)��һ�����Ŀռ�ṹΪƽ�������Σ���̼ԭ��Ϊsp2�ӻ�������̼ԭ���µ��Ӷԣ���˼۲���Ӷ���3����ѧʽΪCH3+���ڶ������Ŀռ�ṹΪ�����Σ���̼ԭ��Ϊsp3�ӻ�������̼ԭ����1���µ��Ӷԣ���˼۲���Ӷ���4����ѧʽCH3-���ʴ�Ϊ��CH3+��CH3-��
(2)�ɵڶ����ڷǽ���Ԫ�ع��ɵ����Է��ӣ��ڶ�����Ԫ��Ϊ����ԭ�ӣ�ͨ��sp2�ӻ��γ����Է��ӣ���ƽ���η��ӣ������ͷ�����BF3���ڶ�����Ԫ��Ϊ����ԭ�ӣ�ͨ��sp3�ӻ��γ����Է��ӣ�����������ͷ��ӣ���÷����м۲���ӶԸ�����4�Һ���һ���µ��Ӷԣ������ͷ�����NF3������÷���Ϊ��������ṹ����÷��ӵļ۲���ӶԸ�����4�Ҳ����µ��Ӷԣ������ͷ�����CF4���ʴ�Ϊ��BF3��NF3��CF4��
(3)SO3��ԭ����Ϊ4���۵�����Ϊ24����SO3��Ϊ�ȵ������ΪNO3-��CO32-��BF3��COCl2�ȣ�NO3-��Nԭ���γ�3��������û�йµ��Ӷԣ��ӻ�����Ϊsp2��̼��������м۲���ӶԸ���=��������+�µ��ӶԸ���=3+![]() (4+2-3��2)=3������ԭ���ӻ���ʽ��sp2��SO3������Sԭ�ӵļ۲���ӶԸ���=��������+�µ��ӶԸ���=3+
(4+2-3��2)=3������ԭ���ӻ���ʽ��sp2��SO3������Sԭ�ӵļ۲���ӶԸ���=��������+�µ��ӶԸ���=3+![]() =3���ӻ�����Ϊsp2���ʴ�Ϊ��NO3-��CO32-��sp2��
=3���ӻ�����Ϊsp2���ʴ�Ϊ��NO3-��CO32-��sp2��


 53���ò�ϵ�д�
53���ò�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��CuSO4��һ����Ҫ����ԭ�ϣ����Ʊ����й�������ͼ��ʾ��
��1����Ҫ����ͼ��ʾ��Ũ���������Ʋ����������Ҫ��1mol/L��ϡ����480ml����Ҫ������Ũ��������Ϊ______ml��
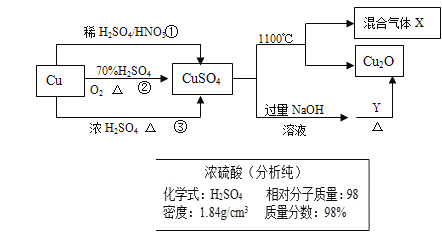
��2�����Ƹ�ϡ�������õ��IJ�������������������Ͳ���ձ����_______��__________��
��3�����в�����ʹ������ҺŨ��ƫ�͵��� _________��
A. ����ת��������ƿ��û��ϴ���ձ�
B. δ��ȴ�����¾�ת��������ƿ
C. ����ƿ�д�����������ˮ
D. ����ʱ���ӿ̶� E.��ȡŨ����ʱ��Ͳ������������ˮ
��4����ȡ����ͭ��;���٢ڢ��У�;��_________�ܸ��õ�������ɫ��ѧ��˼�롣
��5������1000ml 0.1mol/L������ͭ��Һ������������ƽ��ȡ________g������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ڹ�ũҵ������Ӧ�÷dz��㷺����ͼ��ʵ�����Ʊ�����������һϵ�����ʵ���װ�á�
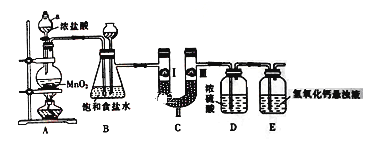
(1) ����a������Ϊ_______________��
(2) װ��B�б���ʳ��ˮ��������_________________��
(3) д��Aװ���з�����Ӧ�Ļ�ѧ����ʽ______________________
(4) װ��C����������֤�����Ƿ����Ư���ԣ�Ϊ��װ��C��I��II��III�����η���__________(����ĸ)��
ѡ�� | a | b | c |
I | ʪ�����ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
II | ��ʯ�� | Ũ���� | ��ˮ�Ȼ��� |
III | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
(5) װ��E��������������Һ��������___________________��ͬʱ��װ�ù�ҵ�Ͽ�������ȡƯ�ۣ���д����Ӧ��Ӧ�Ļ�ѧ����ʽ��___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼΪCaF2��H3BO3(��״�ṹ�����ڵ�H3BO3����ͨ��������)������ͭ���־���Ľṹʾ��ͼ����ش��������⣺
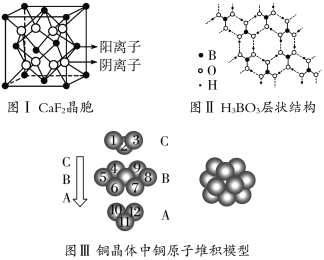
(1)ͼ����ʾ��CaF2��������Ca2������ҵȾ����F����Ϊ________��ͼ����δ��ŵ�ͭԭ���γɾ������Χ����ڵ�ͭԭ����Ϊ________��
(2)ͼ����ʾ�����ʽṹ�������ܲ��Ѵ�8���ӽṹ��ԭ����________��H3BO3������Bԭ�Ӹ����뼫�Լ�������Ϊ________��
(3)���־������۵���͵���________���侧�������ۻ�ʱ���˷�����֮��������Ϊ________________________________________________________________��
(4)���CaF2����ľ���ʾ��ͼ����֪���������������Ca2���˼����Ϊa��10��8 cm������CaF2������ܶ�Ϊ________g��cm��3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з����У�����ԭ�ӵ��ӻ����������ͬ����
A. CO2��SO2B. CH4��NH3
C. H2O2��C2H4D. C2H4��N2H4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��Q��E����Ԫ���У�Xԭ�Ӻ����M����ֻ�����ԳɶԵ��ӣ�Yԭ�ӵĺ���L���������K���������Z�ǵؿ��ں���(��������)��ߵ�Ԫ�أ�Q�ĺ˵������X��Z�ĺ˵����֮�ͣ�E��Ԫ�����ڱ��ĸ�Ԫ���е縺�������ش��������⣺
(1)XZ2�ķ���ʽΪ__________�����ӵ����幹��Ϊ__________��
(2)YZ2�ĵ���ʽΪ__________��������к���__________��![]() ����__________��������
����__________��������
(3)Q��Ԫ�ط�����__________������__________�������ĺ�������Ų�ʽΪ__________���۵����Ų�ͼΪ__________�����γɻ�����ʱ����ߵĻ��ϼ�Ϊ__________��
(4)E�ĵ�����ˮ��Ӧ�Ļ�ѧ����ʽΪ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ�Ͽ����÷���м�Ʊ�����Fe3O4����������ͼ��
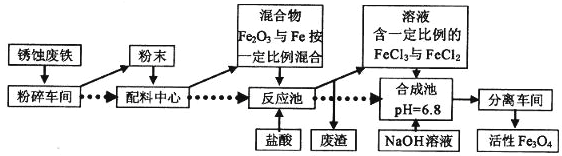
��1�����Ʊ������У�Ҫ����״����ԭ�Ϸ��顢ĥ�ɷ�ĩ��������________________________
��2���ںϳɳ�������Fe3O4�����ӷ���ʽΪ__________________________
��3�������ڷ�Ӧ���м���û���������,�������̿�֪���������ĺܿ���ʹ������е�Fe2O3��Fe���ʵ���֮�Ƚӽ�________
��4��ijͬѧ���÷���м����Fe��Fe2O3������ȡFeCl3��6H2O���壬ͬʱ�ⶨ���������������������װ����ͼ���г�װ���ԣ��������Ѽ��飩��

�����������£�
I�����ɼ�K1���رյ��ɼ�K2��������a�������μ����ᡣ
������ʱ���رյ��ɼ�K1���ɼ�K2����A����Һ��ȫ�����ձ���رջ���a��
���ձ�����Һ����Ũ������ȴ�ᾧ�����˺�õ�FeC13��6H2O���塣
��ش�
�ٲ������С���������������______________���ձ��е�������________________����Ӧ�ķ���ʽ��________________��________________���������ӷ�Ӧ��д���ӷ���ʽ��
�������������Ϊm g��ʵ���������B�����õ�������V mL����״��ʱ������ͬѧ�ɴ˼�����˷���м����������������![]() ������ֵ��ʵ����ֵƫ�ͣ���ʵ����̲�������ƫ�͵�ԭ����______________________��
������ֵ��ʵ����ֵƫ�ͣ���ʵ����̲�������ƫ�͵�ԭ����______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����״���£�V L HCl�ܽ���1Lˮ�У�ˮ���ܶȽ���Ϊ1g/mL����������Һ���ܶ�Ϊ�� g/mL����������Ϊw�����ʵ���Ũ��Ϊc mol/L�������й�ϵ�в���ȷ�ģ� ��
A.![]()
B.![]()
C.![]()
D.![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ѧ������ʵ���е�Ӧ���������֪ʶ��ա�
��1������6��02��1023����ԭ�ӵ�H2SO4�����ʵ�����___________��
��2�����״����V LCO2������ԭ����Ŀ��ͬ��ˮ��������___________g���÷�ʽ��ʾ��
��3����NAΪ�����ӵ���������ֵ�����a g�����к��еķ�����Ϊb����c g�����ڱ�״���µ����Լ��________(�ú�NA��ʽ�ӱ�ʾ)��
��4����4 g NaOH�ܽ���ˮ�����10 mL��Һ����ϡ�ͳ�1 L������ȡ��10 mL����10 mL��Һ�����ʵ���Ũ��Ϊ_______��
��5�������Ϊ1��2��3���Ȼ��ơ��Ȼ�þ���Ȼ�����Һ���ֱ������������Ũ�ȵ���������Һ����ǡ����ȫ��Ӧ�����Ȼ�������������������Һ�����ʵ���Ũ��֮��Ϊ_________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com