��2008?����һģ��̼���⡢��3��Ԫ����ɵ��л���A����Է�������Ϊ102���������������Ϊ9.8%����������ԭ�Ӹ���Ϊ����5����
��1��A�ķ���ʽ��
C5H10O2
C5H10O2
��
��2��A��2����ͬ�ĺ��������ţ��������������
�ǻ���ȩ��
�ǻ���ȩ��
��
��3��һ�������£�A��������Ӧ����B��B���ӵĽṹ����Ϊ1��̼ԭ��������2����������2���ṹ��ͬ�Ļ��ţ� ��A�Ľṹ��ʽ��
��
��A���ܷ����ķ�Ӧ�ǣ���д�����ĸ��
b
b
��
a��ȡ����Ӧb����ȥ��Ӧ c��������Ӧd����ԭ��Ӧ
��4��д��������A������ͬ�����š�������֧����ͬ���칹��Ľṹ��ʽ��
��
��
��5��A������һ������ͬ���칹�壬���칹��������������ˮ�⣬����������Է���������ͬ�Ļ��������һ�ֵķ�������2�������˷�Ӧ�Ļ�ѧ����ʽ��
CH
3COOCH��CH
3��
2+H
2O
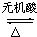
CH
3COOH+��CH
3��
2CH-OH
CH
3COOCH��CH
3��
2+H
2O
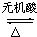
CH
3COOH+��CH
3��
2CH-OH
��







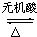 CH3COOH+��CH3��2CH-OH
CH3COOH+��CH3��2CH-OH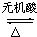 CH3COOH+��CH3��2CH-OH
CH3COOH+��CH3��2CH-OH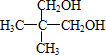 ����ӦΪ
����ӦΪ ��
��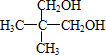 ����AΪ
����AΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� ��
�� ��
�� ��
�� ������������Ҳ�ɣ���
������������Ҳ�ɣ��� CH3COOH+��CH3��2CH-OH���ʴ�Ϊ��CH3COOCH��CH3��2+H2O
CH3COOH+��CH3��2CH-OH���ʴ�Ϊ��CH3COOCH��CH3��2+H2O CH3COOH+��CH3��2CH-OH��
CH3COOH+��CH3��2CH-OH��

 ѧ���쳵�����ּ��ں�����ҵϵ�д�
ѧ���쳵�����ּ��ں�����ҵϵ�д� ��˼ά������ҵϵ�д�
��˼ά������ҵϵ�д�





 +O2
+O2 +2H2O
+2H2O +O2
+O2 +2H2O
+2H2O
