
A��B��C��D��E��F�������ʵ��ת����ϵ��ͼ��ʾ����Ӧ���������ֲ���δ�г�����
��1����A�dz����������ʣ���B��ˮ��Һ��Ӧ����C��D��D��F�����嵥�ʣ�D��F��ȼ��ʱ������ɫ���档��F����Ӧ��Ԫ�������ڱ�λ���� ����Ӧ�ڣ���ˮ��Һ�н��У������ӷ���ʽΪ ��
��2����A��DΪ������Ԫ����ɵĹ��嵥�ʣ�AΪ������DΪ�ǽ������Ңۢ�������Ӧ���к���ɫ�������ɣ���Ӧ�١��ܵĻ�ѧ����ʽ�ֱ�Ϊ
�� ���� ��
��3����A��D��F���Ƕ����ڷǽ������ʣ���A��D����Ԫ��ͬ���壬A��F����Ԫ��ͬ���ڣ�C��һ������Ѫ�쵰��ϵ��ж����壻������B�ľ��������� ������E�Ľṹʽ�� ��
��1����4���ڣ��ڢ��� 2Fe2++Cl2=2Fe3++Cl-
��2��2Mg+CO2 2MgO+C�� C+2HNO3(Ũ)=CO2��+2NO2��+2H2O��
2MgO+C�� C+2HNO3(Ũ)=CO2��+2NO2��+2H2O��
��3��ԭ�Ӿ��壬 O=C=O��
���������������1��������Ϣ�ж�AΪ����BΪ����������ڱ��е�λ���ǵ�4���ڣ��ڢ��壻��Ӧ�����������ӱ�����������2Fe2++Cl2=2Fe3++Cl-��2��������Ϣȷ��AΪþ��DΪ̼��FΪŨ���ᣬ���Է�Ӧ��Ϊ2Mg+CO2 2MgO+C����Ӧ��ΪC+2HNO3(Ũ)=CO2��+2NO2��+2H2O��
2MgO+C����Ӧ��ΪC+2HNO3(Ũ)=CO2��+2NO2��+2H2O��
��3��CΪCO��AΪ̼��BΪSiO2��FΪ������SiO2Ϊԭ�Ӿ��壬CO2�ṹʽΪO=C=O��
���㣺����ͼ���⣬�漰���ʵ�ת����ϵ�й����⡣


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��һ�ֺ���ɫ���������B��D�ǽ������ʣ�J��һ��������ˮ�İ�ɫ��������Ⱥ��������ֽ⡣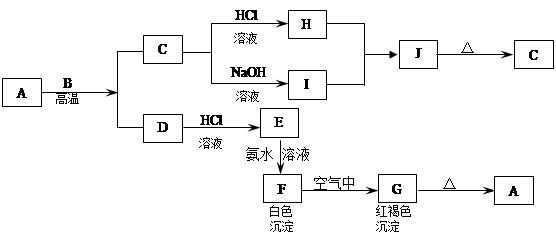
��1��A�Ļ�ѧʽ�� ��
��2��F�ڿ�����ת��ΪG�Ļ�ѧ����ʽ�� ��
��3��Cת��ΪI�����ӷ���ʽ�� ��
��4��Dת��ΪE�����ӷ���ʽ�� ��
��5��D�ڸ����º�ˮ������Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ʾ����֪��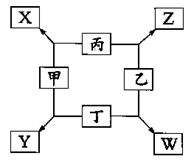
�ټס��ҡ���������Ϊǰ������Ԫ�صĵ��ʣ��ס��ҡ� ��������Ϊ���塣
����һ�������¼�����ͼ��붡����������֮��l��3��Ӧ���ֱ�����X��Y���ڲ�����Ԫ�ؼ׳ʸ��ۡ�
����һ������������������붡�������ʵ���֮��1��2��Ӧ���ֱ�����Z��W���ڲ�����Ԫ���ҳʸ��ۡ�
����գ�
��1��W�ĵ���ʽΪ��������������
��2��X���Ҵ������Ļ�ѧ����ʽ��______________________________________________��
��3��Y��Z��Ӧ�Ļ�ѧ����ʽ��_____________________________________________��
��4��2.4g�����������ҷ�Ӧ����W�ų�QkJ���ȣ���÷�Ӧ���Ȼ�ѧ����ʽΪ ��
��5��ʵ������ȡ�������ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��A��J��Ϊ��ѧ��ѧ�г��������ʣ�����֮��������ת����ϵ������A��DΪ�������ʡ�����Ӧ���������ɵ�ˮ��������������ȥ��
��ش��������⣺
��1��B��_____________��H��_____________���ѧʽ��
��2��д��J��D��Ӧת��ΪG�����ӷ���ʽ_______________________________________
��3��A�ڳ�����Ҳ����NaOH��Һ��Ӧ����F��д���˷�Ӧ�����ӷ���ʽ _____________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ҺA������ҺB�����³������ʵ�飬�������������жϣ�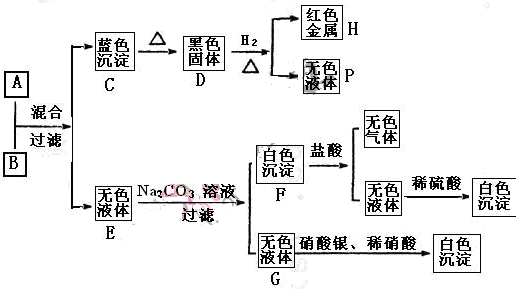
��1��A�Ļ�ѧʽ B�Ļ�ѧʽ ��
��2���������ת���Ļ�ѧ����ʽ�����á������ŷ�����������ת�Ƶķ������Ŀ��
D+H2��H+P��
��3�� д�����з�Ӧ�����ӷ���ʽ��
A��B��
F�����
��4������ҺB�������ӵļ��鷽����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��Ϊ��ѧ��ѧ�������ʣ��Һ���һ����ͬ�� Ԫ�أ�����֮��������ת����ϵ������A�ǵ��ʡ�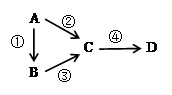
��1����A��һ�ֵ���ɫ���壬B����̬�⻯�C��DΪ��̬�������C���γ��������Ҫ���ʡ���д��C��B��Ӧ�Ļ�ѧ����ʽ_________________________________��
��2����B����̬�⻯�C��DΪ��̬�������C��D���γɹ⻯ѧ������һ����Ҫԭ����д����Ӧ�۵Ļ�ѧ����ʽ____________________________________��ʵ�����м�������B�����õ��Լ�����ƷΪ________________��
��3����B��D��������ǿ����Һ����������ǿ����Һ����Ӧ�ڢ۾���Ҫǿ������Һ����Ӧ�ܿ���ͨ���μ�����ϡ����ʵ�֡��ݴ��ж�AԪ�������ڱ��е�λ����_________________����д���ڵ����ӷ���ʽ___________________________________��
��4����C��һ�ֵ���ɫ���壬��������������еĹ�������D��һ��ǿ�д����Ӧ�ܵ����ӷ���ʽ___________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����֪A��B��C��D����ѧ��ѧ�ij������ʣ���A��B��C������ͬһ��Ԫ�ء���һ������������֮����ת����ϵ��ͼ��ʾ(���ַ�Ӧ�е�H2O����ȥ)��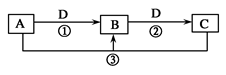
��ش��������⣺
��A����������ˮ������D�����������������������;���Ľ�������
�ټ�������B����Һ���ܵõ�B����B�Ļ�ѧʽ������________________����ҵ����ȡA�����ӷ���ʽΪ__________________________________��
����A�Ʊ�Ư�۵Ļ�ѧ����ʽ��________________________________________________����Ӧ�ڵ����ӷ���ʽ��______________________________________________________,����C����Һʱ�ɼ���������______�����������ƣ�������ˮ�⡣
��2����A��ijǿ���ϡ��Һ,��A�Ļ�ѧʽ������________��
��3����D���ȼҵ����Ҫ��Ʒ֮һ��B�����ԣ���Ӧ�ڵ����ӷ���ʽ��________________________��
��4����A��B��C�ĵ���ɫ��Ӧ���ʻ�ɫ��D����̬�����������D������______��______���ѧʽ����������������̬���������ﳣ��ѡ��________________����һ���Լ����ƣ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ɶ�����Ԫ����ɵĵ���A��B��C�ͼס��ҡ����������ֻ���������ͼ��ת����ϵ����֪CΪ�ܶ���С�����壬��Ϊ�ǵ���ʡ�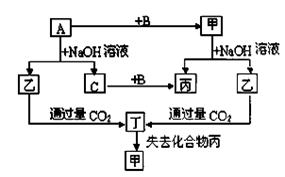
��������ת����ϵ�ش��������⣺
��1��д���������ʵĻ�ѧʽ��A B �� ��
��2��Ԫ��A�����ڱ��е�λ���� ��д����һ ����; ��
��3��д�����б仯�ķ���ʽ��
A��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ
�������CO2��Ӧ�����ӷ���ʽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����ʵ���У����ܹ۲쵽���Ա仯���ǣ�������
| A����һС�δ�ĥ������Ƭ����������ˮ�� | B��������ͨ��FeCl2��Һ�� |
| C�����̶���ļ�Ͷ��ʢ�ڴ��ձ��ڵ�ˮ�� | D������ˮ�μӵ�KI������Һ�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com